Researchers at the University of Pennsylvania, Philadelphia, have adapted self-assembling membranes for more-energy-efficient nanofiltration in organic solutions such as ethanol and isopropanol. These membranes are compatible with organic solvents and can be tailored to address different separation challenges. Organic solvent nanofiltration can reduce the footprint of traditional thermal separation processes, note the researchers, who add the uniform pore size of the membranes they developed provide compelling advantages in terms of membrane selectivity and, ultimately, energy efficiency as well.
Their study, published in Science Advances, describes how the uniform pores of the membrane can be fine-tuned by changing the size or concentration of the self-assembling molecules. The researchers believe this tunability enables the membrane technology to help solve more diverse real-world organic filtration problems.
“The membranes provide the ability to change the pore size in small increments, and also the surface chemistry of the pore walls. Some of the real-world organic filtration problems of interest include acetone recovery, homogeneous catalyst recovery, recovery of hexane and ethanol used in solvent extraction, and aromatic enrichment (e.g., separation of benzene from cyclohexane, as model systems). We anticipate that tuning of the pore size and surface chemistry will make it possible to tailor the membrane performance for the particular applications of interest,” says Chinedum Osuji, a professor in the school’s Department of Chemical and Biomolecular Engineering.
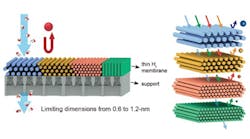
Maintaining membrane stability in organic solvents with different polarities posed a challenge. To combat this, the team created membranes by first forming lyotropic mesophases of surfactants in water, spreading the soft gel as a thin film, and then using a chemical reaction to link the surfactants together to form a nanoporous polymer. The size of the pores in the polymer are set by the self-assembled structure of the lyotropic mesophase.
“At a certain concentration in an aqueous solution, the surfactant molecules aggregate and form cylindrical rods, and then those rods will self-assemble into a hexagonal structure, yielding a gel-like material,” explains Osuji. “One of the ways we can manipulate the permeability, or size of the pores in our membranes, is by changing the concentration and size of the surfactant molecules used to create the membrane itself. In this study, we manipulated both of those variables to tune our pore sizes from 1.2 nanometers down to 0.6 nanometers.”
“A specific application for this technology is biofuel production,” notes Osuji. “We are working on separations of model mixtures, and specifically, on examining separation of butanol-water. This is work that is currently in progress in the lab,” he adds.
Moreover, because the manufacturing of many pharmaceutical products requires the transfer of a chemical intermediate from one solvent to another miscible solvent, this new membrane may provide a perfect solution to drug development filtration needs, say the researchers.
The team also notes that larger-scale membrane fabrication can use roll-to-roll solution processing rather than the current solution-based spin-coating method.
“Spin-coating is a solution-based fabrication process, as is roll-to-roll processing. Industrial-scale membrane fabrication typically uses roll-to-roll processing, with dip coating or blade coating for deposition of material from solution onto a large support film. We need to translate our current process conditions (defined by solution concentration, spin speed and spin duration) into conditions that yield the same result for a roll-to-roll compatible deposition. (e.g., concentration, blade height, film speed),” Osuji further explains.
“We are conducting experiments on the mechanical properties of the selective layer materials, but in practice, the durability in service is what is important,” stresses Osuji.
Furthermore, the membrane’s chemistry (rich in quaternary ammonium groups) makes it robust against bio-fouling, say the researchers. “We have not examined other types of fouling or scaling behavior,” he notes.
“The technology we are pursuing is opening up fundamentally new possibilities for rationally designed uniform nanostructured membranes. We are excited about the potential to affect a broad range of selective transport applications,” concludes Osuji.