Plant InSites: Why Kitchen Logic Doesn’t Transfer to Industrial Heat
In our daily lives, we know that if we heat something on the stove for a longer period, it will become hotter.
Experience with daily life may not always give us clear experience to use in troubleshooting plant operations. Heat transfer is one area where daily experience around the house and garage may lead us astray unless carefully thought through. Most processes in daily life where we heat something are essentially batch processes rather than continuous ones. Now, if your plant specializes in only batch processes, the following discussion may not directly apply. Still, most larger-capacity process plants use continuous processes.
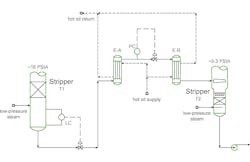
In the July Plant InSites column, I discussed issues with two control valves in series at a chemical plant. To recap, at higher rates, the plant struggled to control the outlet temperature from exchanger E-B into the downstream stripper. The temperature was dropping and not enough of the feed to T2 could not be vaporized (Figure 1).
One operator asked, “Why not use the PC valve to pinch back on the flow out of exchanger EA and keep the process in the exchanger longer? That will let the exchanger heat up the process more.” This is a specific case where our day-to-day batch heating experience can lead to confusion when evaluating a continuous process.
In our daily lives, we know that if we heat something on the stove for a longer period, it will become hotter. This is because cooking is mostly a batch process. More time leads to a higher cooking temperature.
This reasoning does not necessarily hold true for a continuous process. In a simplified form, heat transfer in a heat exchanger is Q = U A ΔTLMTD-Corrected. Q is duty, U is overall heat transfer coefficient, A is area and ΔTLMTD-Corrected is the temperature difference adjusted for exchanger geometry and flow patterns.
Neither A nor ΔTLMTD-Corrected includes time as a factor. U is a bit more complicated. Convective heat transfer varies with fluid velocity (or Reynolds number). For a given exchanger, a higher velocity will change the time the fluid is in the exchanger. However, the determining factor is velocity. Velocity changes both the heat-transfer coefficient and the time the process was in the exchanger. Changes in time in this process are not causes of changes in heat transfer. In fact, while two-phase heat transfer can hide much complexity, in this service, higher velocities and lower residence times, increase the amount of heat transfer.
Trying to pinch back on the pressure controller and retain liquid longer in exchanger E-A is completely the wrong approach. First, the plant wants to keep the production rate as high as possible. If the upstream level is under control, the time cannot be changed in E-A very much because the flow rate is set by the level controller. Residence time in E-A might be extended due to suppressing vaporization in E-A, but this is counterproductive. Keeping E-A in sensible heat service (no vaporization) reduces the heat-transfer coefficient and reduces the duty of the exchangers, reducing the vaporization of T2 feed.
After some discussion of the principles involved, the senior operator appeared unconvinced.
I countered that he could “just try it and see what happens.”
In follow-up discussions with the plant on other matters the following week, the subject didn’t come up again. I’m not sure if they ever tried the recommended approach, but in any case, the plant remains a consistent client.
About the Author
Andrew Sloley, Plant InSites columnist
Contributing Editor
ANDREW SLOLEY is a Chemical Processing Contributing Editor.