This Month’s Puzzler
An engineering firm we hired disagrees with me about pressure relief on a methanol/water/formaldehyde column (see figure). The company is adamant that we must size and install a relief valve for two-phase flow.
The distillation tower operates at about 15 psig and has a design pressure of 45 psig. The tower recovers at the condenser about 99% of the methanol along with a few percent of formaldehyde and water. The methanol gets recycled back to the formaldehyde plant. Some trace vapor travels through 110 ft of 1-in. pipe before passing into a 16-in. vent to a thermal oxidizer (TOX).
After I heard from the firm’s engineer whose analysis indicated the relief valve was necessary, I did a fire-sizing calculation myself and then computed the choked flow for the 1-in. vent from the receiver to the TOX knockout drum. I found the choked flow capacity far exceeds that required for fire sizing. My engineering report disparaged the loss of methanol to the TOX but concluded that no relief valve is needed.
The firm’s relief expert counters that I can’t take a reduction for four inches of fiberglass insulation in a stainless steel jacket; he says fiberglass may burn. Without that reduction, the flow capacity is much greater than the choked flow. Besides, he contends, two-phase flow, not vapor, will exist in the 1-in. vent. He also notes that he must look at other scenarios. Initially, he challenged my engineering report by mentioning the vent line could have an isolation valve — thus, necessitating a relief device. However, the line to the knockout drum doesn’t have a valve. He also stated the relief must be on the tower, not the receiver.
In addition, the expert says the vent system might not be capable of handling the flow of all the vessels connected to it if a site-wide fire occurred. I told him we don’t assume the entire site is on fire to size a vent for a relief flow.
What do you think? Do we need a relief valve on this distillation tower? Is the 1-in. vent adequate? Are there any other scenarios worse than a pool fire that could result in a large relief flow? Is two-phase flow in the vent likely or is it just something the expert wants to consider to reinforce his argument?
Review Relief Scenarios
Briefly put, whether a need exists for a relief valve is determined by considering various scenarios and the possibility of exceeding the design pressure or maximum allowable working pressure. API-521 suggests you consider various scenarios, including for example: a) failure of cooling medium; b) failure of protective instruments and interlocks (safety instrumented functions); c) external fire; d) blocked outlets; and e) maintenance scenarios, where applicable.
If we consider loss of cooling water flow to the condenser when the column is at 100% operation, the temperature of the overhead vapor could get close to the temperature of 50-psig saturated steam (≈292°F). The vapor pressure of methanol could reach close to 130 psia — much higher than the design pressure of the column (45 psig). You need to compare the vapor flow rate and the choked flow (from the column to TOX). If the vapor generation exceeds choked flow, the column could develop pressures higher than the design pressure and a relief valve would be necessary on the column.
Regarding the issue of fire case, risk analysis methods suggest that you don’t give credit to protection layers such as insulation. Typically, fire case valves tend to be smaller size than those that consider blocked flow.
As for the relief valve on the line to TOX, although there would be only vapor flow, a high level in the receiver could send two-phase flow to the relief valve. Unless there is adequate liquid disengagement space, the relief valve could be subjected to two-phase flow.
Choose the location of the relief valve carefully. There must be no block valves between the column and receiver. Check the scenario that could generate vapor in excess of choked flow; this would act like a blocked valve, which will increase pressure in the column. The bottom line in some situations is your company’s risk tolerance criteria, i.e., the level of protection (against overpressure) desired by the company.
GC Shah, senior advisor
Wood Group, Houston
Consider Four Key Questions
When it comes to distillation towers, you always want to be certain that you put the right pressure relief device (PRD) in the right spot. Due to the complexities of distillation towers, many relief cases can exceed the normal relief rates you see from your typical vessel overpressure scenarios. In events such as these, it’s always good to get an experienced evaluator or a second opinion of the evaluation of your column PRDs.
Do we need a relief valve on this distillation tower? In short, yes. For now, we can say that either the current relief device must be moved to the distillation tower or that you must install a separate PRD on the tower.
The first problem that I noticed when evaluating this system is that the current relief device can be isolated from the distillation column during failure/flooding of the tower condenser. For example, let’s evaluate a blocked liquid outlet on your overhead receiver. The next event to occur would be that your receiver overfills. Afterward, the excess liquid would spill into your cooling/condenser line, thereby blocking your distillation tower from being able to remove its vapor contents. Your tower would begin to overpressure as your reboiler continues to generate vapor that can’t be relieved due to the flooding in your overheads. At this point, liquid displacement would begin occurring as your tower vapors begin pushing out liquid from the flooded receiver. Note that if your 1-in. vent can adequately relieve this liquid displacement scenario, it possibly can remain as your primary relief device. However, from experience, the 1-in. vent line is unlikely to relieve liquid displacement.
Is the 1-in vent adequate? This is an open-ended question that depends on the unit process, fluid composition and applicable overpressure scenarios. However, a general answer can be found in the response to your next question…
Are there any other scenarios worse than pool fire that could result in a large relief flow? It depends on the original engineering design of this column. It’s possible this system was designed for a method where there are no means for this vessel to overpressure outside of a pool fire. However, many times this isn’t the case for older process facilities. In this likely event, it’s very possible several overpressure scenarios could create relief flows much greater than those caused by pool fire, e.g., power failure, loss of cooling leading to blocked vapor outlet, etc. In such cases, it’s hard to imagine that a 1-in. vent would adequately relieve the typical flow that we see column scenarios generate in comparison to pool fire scenarios.
Is two-phase flow in the vent likely or is it just something the expert wants to consider to reinforce his argument? Two-phase flow is a legitimate possibility; however, the certainty of it can’t be considered without further evaluation. An overpressure scenario that is acknowledged by the Design Institute for Emergency Relief Systems, known as “liquid disengagement,” is an event that can likely occur in receivers and drums such as the one on the overhead of your column, which would result in a two-phase flow.
Adam Agha, PSM consultant
Provenance Consulting, LLC, Houston
Venting Looks Valid
Choked flow for two-phase flow is difficult to calculate. I don’t think the flow is anything but vapor given the disengagement volume available. You could ask the firm how they plan to calculate the pressure drop through the 1-in. vent line.
As for other scenarios, other than a runaway steam flow to the reboiler, I can’t see another scenario that exceeds the flow rate from a pool fire. And I don’t see the runaway control valve resulting in two-phase flow, only vapor flow.
The firm’s expert may have a point on the insulation. Although API-521, 5.15.2.2 allows a reduction in the environmental factor, F, from insulation, the standard requires that the insulation survive a fire hosing. Most insulation, including otherwise stout stainless steel straps won’t survive that kind of abuse. Then there’s the insulation jacket. Stainless steel will survive the temperature API developed from experiments, 1,400°F. However, aluminum won’t; it melts at about 1,220°F, maybe lower, maybe higher depending on alloy composition. Also it’s worth mentioning that a non-glossy jacket won’t radiate heat away like a new stainless steel jacket.
Although fiberglass insulation will begin to melt about 2,100°F, it won’t burn. By the time it gets close to this temperature it likely will lose its insulation capability but then the stainless steel probably will start to discolor and become less reflective as it heats up.
The bottom line is that you don’t want to be in the wrong: run your decision to accept allowance for the insulation by the corporate insurance company.
What about the methanol loss? Perhaps it would be worth sizing a relief valve for economic reasons.
Dirk Willard, consultant
Wooster, Ohio
October’s Puzzler
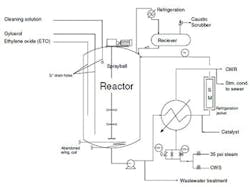
Our ethylene oxide (EtO) batch reactor (Figure 1) suffered an explosion one Saturday morning. The reactor was filled with glycerol before EtO feeding began. A steam-heated exchanger raised the temperature of the reactor to 240°F. The pressure spiked suddenly when the EtO addition started; the operator had been taught that meant the EtO wasn’t reacting. The reactor had been cleaned the night before with water and caustic.
Figure 1. Significant damage occurred after opening the steam block valve.
The shift supervisor authorized the operator to raise the trip point to 400°F and to continue to heat the reactor. The supervisor, who had experience in a similar process where the temperature sensors became fouled and insulated, didn’t trust the temperature reading. Besides, the night foreman had steamed the purge nitrogen in the insulated reactor a half hour before glycerol was added; this isn’t part of the cleaning procedure, just something operators have done for years.
Recent changes in the control system faceplate weren’t fully understood yet. The operator, confused by the labels for the cooling and heating block valves (that feed the temperature control valve), decided to take a walk outside. The faceplate didn’t indicate the pump was circulating through the heat exchanger and catalyst bed all along, which the operator confirmed in the field. However, instead of finding the cooling water valve open and the steam valve closed as expected, both valves were closed. The supervisor ordered the operator to open the steam block valve; the reactor relief valve blew but not before two flanges on the reactor split, damaging the reactor and sending parts of the top reactor pipe flying.
After the accident, the new supervisor blamed the operator for opening the valve. An inspection of the reactor also showed suspicious holes near the top of the reactor in the dip tubes for the caustic, glycerol and EtO.
The superintendent, who wasn’t aware the reactor contained an abandoned refrigeration coil, blamed it for the incident, saying a hole allowed caustic to collect in the coil and that the caustic leaked out and led to a pressure spike when the EtO reacted with it. The superintendent asked a retired operator who knew about the coil and the dip tubes why the caustic never caused this problem before. That person responded that in the past the reactor was flushed by hot water before being purged and dried. Cutback in inert gas usage and limits on water consumption led to recent changes in the cleaning sequence.
What do you think the root cause of the incident was? Could we have prevented this accident?
Send us your comments, suggestions or solutions for this problem by September 13, 2019. We’ll include as many of them as possible in the October 2019 issue and all on ChemicalProcessing.com. Send visuals — a sketch is fine. E-mail us at [email protected] or mail to Process Puzzler, Chemical Processing, 1501 E. Woodfield Rd., Suite 400N, Schaumburg, IL 60173. Fax: (630) 467-1120. Please include your name, title, location and company affiliation in the response.
And, of course, if you have a process problem you’d like to pose to our readers, send it along and we’ll be pleased to consider it for publication.