This Month’s Puzzler
A few months ago, we installed a bank of ion exchange (IX) columns to polish our wastewater before it goes to the city treatment plant. (See Figure 1) We have a plating bath operation where we are trying to reduce the Cr+6 to our allowable limit of 0.08 mg/L; the Safe Drinking Water Act sets a maximum of 0.1 mg/L. We use a sedimentation process upstream of the IX columns to eliminate most of the Cr+6. This sedimentation process worked well except for the surges caused by increased flushing of the baths due to solids’ precipitation. The IX columns performed well for several weeks but then we started seeing problems. We’ve experienced high pressure drops, fouling in our backwash tanks and the loss of the column feed pump once. Moreover, we’ve had to replace our cation IX columns already. The city has heard of our problem and expressed concern about us achieving our allowable Cr+6 limit. Any ideas on what’s going on?
[callToAction]
Add Filters
I recommend installing a specialized filtration system especially designed for your wastewater after the sedimentation step. Then use the Cr+6 ion exchangers as the polishing step before release to outfall.
Kevin Minissian, CEO
Norchem Corporation, Los Angeles
Take Several Steps
I have some suggestions and questions:
1. It looks like the feed is too concentrated, exceeding the solubility limit. This is causing precipitation and scaling. This can cause fouling and high downstream pressure drops. You might consider diluting the feed solution in the lab first to see if the problem disappears.
2. Check the quality of your water. Is it scaling or aggressive?
3. High temperature promotes scaling. Consider cooling.
4. Your process works better with proper chemical treatment, i.e., anti-scalants and anti-foulants following the manufacturer’s specifications. So, what is your treatment level?
5. How often do you backwash?
6. What is the permeate rate, flux and backwash rate?
7. Replacing the cation ion exchanger means that backwash could not restore performance and cation is the worst culprit.
8. Lastly, what is the purity level of the backwash chemicals? At what concentration is HCl used?
Dennis Omenka, process engineer
PetroGas Systems Engineering Ltd., Lagos, Nigeria
Look At Backwashing
Ion exchange (IX) columns are prone to several problems: 1) fouling; 2) blinding; 3) high concentrations of ions resulting in a rapidly depleted bed; and 4) poor backwash design.
Blinding often is connected with a poor backwash design. Backwashing is the key to maintaining healthy columns. Aeration, part of the process of lifting the IX bed during backwashing, can oxidize ions, making them more difficult to remove during backwashing. Because you’re dealing with a new system, you will want to look at how this lifting occurs: it should be vigorous but not so much that it disturbs the column. Fe+2 provides a good example of the potential adverse impact — it is highly soluble in water but, after aeration, becomes Fe+3, which is more easily precipitated inside the beads of the membrane. First, check the backwash rates: a strong acid (cation) wash should be at 4–10 gpm/ft2 of bed; a strong base (anion) wash should be about 4–8 gpm/ft2.
High concentrations of ions can cause breakthrough. The higher the ion charge, the more abrupt the breakthrough is. Cr+6 forms a complex ion structure with water, making it even more difficult to predict. Surge flows or high concentrations of Cr+6 will make breakthroughs very sudden. Fortunately, there’s a large storage tank between the sedimentation and IX columns.
Problems with unexpected ions sometimes can be countered with modifications of the backwash. For example, phosphoric acid has been added to water softening to get rid of copper, which is aerated from Cu+1 to Cu+2, which can cause blinding in columns. Another modification is sodium hydrosulfite, sodium bisulfite or citric acid to unblind iron from media. You may find guidance in the technical literature but still should run a bench-scale trial with the water to confirm changes to the backwash.
I think fouling is the likely culprit, although a thorough analyst will check for other causes. Generally, flocculent settlers require a filtration step afterwards. This is especially true with a membrane unit such as an ion exchanger. Somehow, you got by for several weeks by collecting the solids in the bottom of the storage tank. I recommend a coarse-strainer/sock-filter combination at the discharge of the pumps feeding the liquor to the storage tanks.
Sock filters are available down to about 0.5 microns. Strainers are limited to about 40 microns. You may want another filter in-between to avoid changing the sock too often; most socks are re-useable and can be set up to backwash. The finer the sock, the more difficult it is to detect a leak. My advice is to run some filtration tests in a laboratory so you can get the coarsest sock that will meet your needs. These tests also will help you identify if the filtrate is friable or hard. Hard material can damage the filter.
Dirk Willard, consultant
Wooster, Ohio
November’s Puzzler
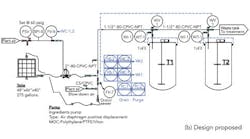
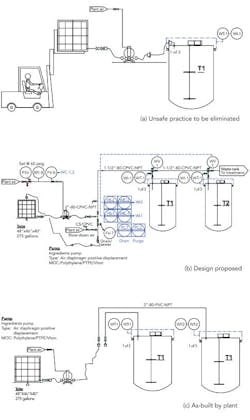
We needed to correct an unsafe practice in our blending area — using a forklift to raise and then hold a tote in place while we pump ingredients into our blending tanks (Figure 1a). Corporate engineering proposed an automated design with six tanks with valves on the top of each tank (Figure 1b) that would cost about $49,000. I thought this was a dumb idea and one for which we’d never get funding anyway, so I had maintenance simplify the design (Figure 1c). We don’t need valves on top of the tank; we’ll control the weight added with a valve near the pump. Because we don’t need valves, we ran individual pipes to each blending tank. What does corporate engineering know anyway?
Unfortunately, we’re having trouble meeting the 1-lb accuracy needed. In addition, we can’t get all the ingredients out of the pipe. Our parking lot is filling up with product rejects. Many ingredients are in the 1–70-cP viscosity range. We tried pumping 990-cP liquid; even the air diaphragm pumps won’t handle it, so we’re hauling it up in buckets.
The corporate engineer says our system won’t work, but the CEO thought it showed initiative at solving the problem on the cheap. The corporate engineer also warns the PVC pipe won’t survive the 180°F water to be used next year for cleaning; his design has CPVC pipe. Is the corporate engineer correct? Did we waste our time?
Send us your comments, suggestions or solutions for this question by October 14, 2016. We’ll include as many of them as possible in the November 2016 issue and all on ChemicalProcessing.com. Send visuals — a sketch is fine. E-mail us at [email protected] or mail to Process Puzzler, Chemical Processing, 1501 E. Woodfield Rd., Suite 400N, Schaumburg, IL 60173. Fax: (630) 467-1120. Please include your name, title, location and company affiliation in the response.
And, of course, if you have a process problem you’d like to pose to our readers, send it along and we’ll be pleased to consider it for publication.
Figure 1. To replace use of forklift to load ingredients (a), plant didn’t adopt setup suggested by corporate engineering (b) but instead went with an arrangement without valves (c).