Revamp Operations Now!
This Month’s Puzzler
At our ammonia plant, a carbon-steel CO2 pipeline exploded during a shutdown. The CO2 gas typically contains 1–3% H2. Our two H2 analyzers, which rely on thermal conductivity, are used to trip valves on the transfer line closed if the H2 concentration rises above 8%; such a concentration poses a risk downstream because it becomes an explosive mixture if combined with air. We use N2 to purge the ammonia plant before a plant outage. We’ve never had an explosion before.
My investigation turned up several issues: 1) operators rely on the trip to close the valves — they’re using a trip as an active control; 2) N2 has twice the conductivity of H2, so operators were instructed to ignore the trip during purging; 3) the analyzers don’t vote and aren’t on the critical check list; 4) the purge is hooked up manually and, because the air-line connection is right next to the N2 one, a wrong hookup is possible — but nobody will admit such a mistake occurred; and 5) the rotometer on the N2 is under-range and the rotometer on the air is over-range. What do you think the source of the ignition was? How do you suggest addressing these issues?
[javascriptSnippet ]
Improve Safety Management
A pattern of poor safety management clearly exists. It’s hard to believe you skirted disaster for so long.
Operators should never, ever, rely on trips to close valves. You need to find out what else they’re doing wrong. Comb through operating procedures, checklists and logbooks — if you have any. Some operators can be fairly clever in covering their tracks, so secure this information right away.
Obviously, your safety strategy has a lot of flaws. First, whenever operators are instructed to ignore an instrument, you need something else or another type of measurement. Second, why are you relying on one type of instrument and doubling down by having two identical forms of measurement? If a form of instrument is unreliable and can’t be replaced, use a voting logic. If the instrument becomes unreliable, it should be backed up by laboratory tests. Here’s another idea: if you can’t accurately measure what’s there, can you measure what’s not? Can you measure the percentage of O2 accurately? Lastly, processes run more smoothly if they are automated. Why isn’t the purge system connected by pipe with automatic valves? A pancake can be installed in a connecting flange to a block valve to ensure no contamination occurs.
If the rotometers had been sized correctly, a field operator might, or might not, have seen the air flow. When instruments can’t measure full range or are measuring over such a range that a measurement can’t be determined, they aren’t very useful.
As for the ignition source, you may never know for sure. It’s possible that rust inside the carbon steel sparked against the wall during the N2 (air) sweep and ignited the fuel/air mixture. In a confined space, the fire became an explosion.
Dirk Willard, consultant
Wooster, Ohio
September’s Puzzler
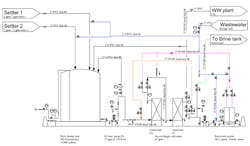
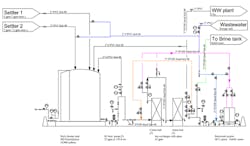
A few months ago, we installed a bank of ion exchange (IX) columns to polish our wastewater before it goes to the city treatment plant. (See Figure 1) We have a plating bath operation where we are trying to reduce the Cr6+ to our allowable limit of 0.08 mg/L; the Safe Drinking Water Act sets a maximum of 0.1 mg/L. We use a sedimentation process upstream of the IX columns to eliminate most of the Cr6+. This sedimentation process worked well except for the surges caused by increased flushing of the baths due to solids’ precipitation. The IX columns performed well for several weeks but then we started seeing problems. We’ve experienced high pressure drops, fouling in our backwash tanks and the loss of the column feed pump once. Moreover, we’ve had to replace our cation IX columns already. The city has heard of our problem and expressed concern about us achieving our allowable Cr6+ limit. Any ideas on what’s going on?
Figure 1. The relatively new system is suffering from a variety of problems.
Send us your comments, suggestions or solutions for this question by August 12, 2016. We’ll include as many of them as possible in the September 2016 issue and all on ChemicalProcessing.com. Send visuals — a sketch is fine. E-mail us at [email protected] or mail to Process Puzzler, Chemical Processing, 1501 E. Woodfield Rd., Suite 400N, Schaumburg, IL 60173. Fax: (630) 467-1120. Please include your name, title, location and company affiliation in the response.
And, of course, if you have a process problem you'd like to pose to our readers, send it along and we'll be pleased to consider it for publication.