Process Puzzler: Relief System Requires Review
This Month’s Puzzler
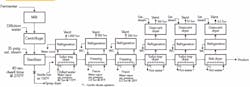
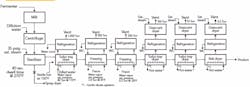
I’m a new-hire production engineer at an ethylene oxide plant. I graduated last year and just finished my probation period, winning my white hat. I went over the production logs a few weeks after I got here and found a real problem: the pressure relief valves (PRVs) keep popping every few months. I wanted to say something right away but, being a new graduate, I didn’t want to question a situation that others already have scrutinized. Recently, though, I talked to the operator on shift at the time of the last PRV release. He said the operators rely on an “automatic” manual vent (one with a lift ball) to prevent the PRVs from opening (see figure).
I then spoke to a few more operators. They all told me they’re so busy since a plant expansion that they have trouble controlling the reactor pressures; sometimes they just let the vent catch the upsets. The PRVs only pop every so often, one said. The foreman advised me drop the matter.
However, I looked more into it and found the manual vent opened every few days and the PRVs have popped about three times a year over the past two years. I can’t get more data because the trends only go back that far. Interestingly, I talked to accounting and discovered we use twice as much sulfuric acid in the scrubber as we did two years ago.
I dug into our files and couldn’t find the sizing calculations for the vent. I don’t think this vent is even legal. What should I do?
[callToAction ]
Check The Relief Scenarios
I certainly share your concern but in no way want to insinuate any wrongdoing by your company. Evidently, the scrubber is installed to vent each reactor on a manual basis and would probably be permitted based on the number of reactor batches in a year and the expected contents of these reactors — assuming very small quantities of reactants. The reaction is probably designed to go to completion with the least environmentally sensitive reactant in excess, protecting the scrubber vent from the most sensitive, such as ethylene oxide.
Such an arrangement of relief devices, including the manual ball vent (note the CSO [car seal open] valves on it in the same manner as the PSVs), in ever-increasing pressures is unusual. I would be more concerned over the relief scenarios of the PSVs. What would these scenarios be to be staged as they are? The goal of a reaction control would be to stay below the 245 psig of the manual vent (manual is a misnomer because it seems to open automatically). That should be done at the reactor inlet end. Pressures approaching the 245 psig should cause a pause of the reaction or lower the rate of the reactant addition. If these are manual additions, the vent ball may be needed for slight excursions but manual relief of the reactor with the worm manual vent should not be used.
Generally, releases in an unusual event going out of a relief device may not be a permit violation. However, the regularity of the reliefs you describe would seem excessive. A PSV should not blow that regularly and, if it has blown, it is usually scheduled for maintenance at the next available outage. It appears that operation of the ball vent is designed to be more frequent or even perhaps with no real deterioration of the device mechanics.
The concern here is that during these vents the rate of reaction will go faster than planned. Hence, the operating pressure will be higher than planned, exceeding the capacity of the scrubber. The net result is potential release of some amount of the constrained reactant, i.e., ethylene oxide.
Better control on the reactant additions is needed, with sufficient documentation of the controls and relief scenarios so the next new engineer will be able to fully understand the operation and safety features of the system.
Tom Brader, staff instrument engineer
Valero, St. Charles, La.
Tread Softly
What was it that Collin Powell said about Iraq? “If you break it, you own it.” Your first step is to determine if there is an upgrade project planned for the reactor vent; if calculation procedures aren’t available from the corporate engineering files, then they should be part of that project. From the figure, it appears that the lifting ball is the first line of defense and was designed before the additional reactors were added. This suggests that it may be at capacity. Did anyone check the capacity prior to the expansion? Obviously, if you suggest something is a problem, then it becomes your burden to solve. There is an alternative scenario: your company or plant is ignoring the problem — and now you’ll be seen as a troublemaker.
As to the question about the legality in the use of the lift ball vent, it’s perfectly safe if there are relief valves protecting the maximum allowable operating pressure. A lift ball is no substitute for a relief valve because it isn’t recognized by the API and OSHA as a safety device.
We ran into this problem on a reactor project I worked on. OSHA wouldn’t budge. They got curious when we mentioned we had a lift ball.
I can tell you one thing that will get you in trouble with regulators: not having design calculations for a lift ball vent. Although it’s not an official relief device, you must have calculations for it — that is what we were told. When I sized the lift ball for higher pressure and flow, I modified calculations for a variable-area flow meter tube, a rotameter: reference rotameters in Noel de Nevers’ “Fluid Mechanics for Chemical Engineers,” 2nd edition, and Richard Miller’s “Flow Measurement Engineering Handbook,” 3rd edition. I also got some help from vendors in establishing flow coefficients. In my case, I additionally had several years of reliable data on lift pressure. Once I reviewed this information, I reverse-engineered the calculation for the existing ball. All of this was useful in designing a larger lift ball to operate at a higher pressure.
Now, let’s consider some safety concerns I noticed when reviewing the figure. The trouble is with the material being handled: ethylene oxide. It polymerizes with itself and actually eats polytetrafluoroethylene — not the light-weight partially fluorinated version but the fully fluorinated polymer. When it polymerizes, ethylene oxide can cause a chain reaction that results in an explosion. Even when it doesn’t cause an explosion, it can gum up valves. As a normal practice, relief devices are removed and replaced if they operate — this is especially important with a material like ethylene oxide. This probably explains why the acid scrubber is consuming large quantities of sulfuric acid. It is likely that the rupture disc is broken under the lift ball. It is even possible that the ethylene oxide reacted with the steel coating the ball to affect its performance by creating additional drag. It is even possible that the ball could eventually fail because it has become corroded to the wall of the vent chute. This could be the reason why the relief valves have been opening more often, or it could be that the relief valves added to the relief header aren’t meeting capacity. In addition, if relief valves are being frequently replaced, there is the risk someday that someone will leave it in and it won’t perform because it’s gummed up. There are some serious questions about the current design of the relief system.
Normally, I advise against adding multiple relief valves. As with spray nozzles in scrubbers, you’re always better with one rather than many. You should insist on a review of the relief requirements.
Dirk Willard, consultant
Wooster, Ohio
June’s Puzzler
We manufacture astaxanthin by fermentation and decided to switch from spray-drying to freeze-drying or lyophilization because freeze-dried astaxanthin is 41% more active than the spray-dried version and can be stored without refrigeration.
A block flow diagram developed by project engineering summarizes the proposed design (Figure 1); the process involves sterilization, low-temperature drying in a turbo tray dryer followed by freezing and drying in a series of tray dryers ending with a belt dryer.
Figure 1. Proposed design has provoked negative comments from a variety of staff.
The design has evoked a lot of criticism. Production complains it’s too complicated and will make it hard to keep the product sterile. Corporate management says we can do away with the sterilization because the freezing process will eliminate bugs. The construction manager argues that maintaining a vacuum between the dryers and even in the dryers themselves will be tough: he suggests going with multiple batch dryers instead. Research counters that the continuous dryers will produce a more-consistent product, even more so than the spray dryers, which are batch. Our safety manager is concerned about dust in the belt dryer. What do you think of the design?
Send us your comments, suggestions or solutions for this question by May 12, 2017. We’ll include as many of them as possible in the June 2017 issue and all on ChemicalProcessing.com. Send visuals — a sketch is fine. E-mail us at [email protected] or mail to Process Puzzler, Chemical Processing, 1501 E. Woodfield Rd., Suite 400N, Schaumburg, IL 60173. Fax: (630) 467-1120. Please include your name, title, location and company affiliation in the response.
And, of course, if you have a process problem you'd like to pose to our readers, send it along and we'll be pleased to consider it for publication.