Don't Let a Second Liquid Phase Faze You
Two liquid phases can form inside distillation towers in specific systems. Typically one phase is ionic, usually aqueous, and the other nonionic, generally oil or hydrocarbon-based. Either phase may be the major component. Water entrapment in hydrocarbon columns is a common example of an ionic liquid phase as the second phase. Fusel oils in ethanol distillation (see: "Cure Column Hiccups") typifies a relatively nonionic liquid phase occurring in a mostly ionic system.
[pullquote]
The leading cause of the second phase is concentration of non-key components. This may stem either from a local composition profile or outright entrapment ("Watch Out for Trapped Components in Towers"). Multiple operating problems can occur with an unwanted liquid phase.
The cheapest method to draw out an unwanted phase usually is a simple liquid sump from a tray. Unfortunately, many towers have ineffective sumps. For the sump to work, the unwanted phase must be the higher-density phase and the sump must be correct.
Often, standard sump layouts are so ineffective that the operating department may simply not believe that a second phase exists — in spite of clear evidence from tower upsets, experience from other plants, and analytic data. After all, if the heavy phase can't be drawn off, it must not be there.
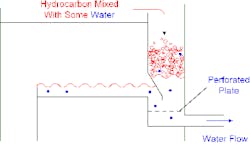
Figure 1. Water draw wouldn't work despite three valiant efforts.
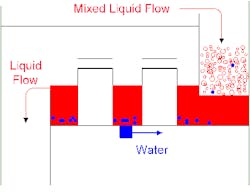
Figure 2. Collector tray enables reasonable draw rate of stream that's mostly water, whose phases then can be separated in an external drum.
ANDREW SLOLEY is a Chemical Processing Contributing Editor. You can e-mail him at [email protected]