Metamaterial Promises to Enhance Separations
An artificially structured material — or metamaterial — made of specially patterned polymers may enable more-efficient, energy-saving membrane separations, believe researchers at the Georgia Institute of Technology (Georgia Tech), Atlanta. They have designed what they claim is the first metamaterial that can sort chemical and biomolecular species.
“With this metamaterial, we can control the direction the atoms can go using the trick of anisotropy,” explains Martin Maldovan, an assistant professor in the School of Chemical and Biomolecular Engineering. “This would be in addition to separation based on solubility and diffusivity. We have added an important parameter to the toolbox of chemical engineers: where to send the atoms.”
[javascriptSnippet ]
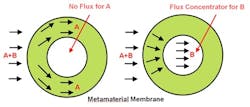
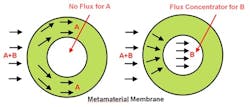
The researchers have performed computational studies on a metamaterial consisting of four different types of polymers, two with high diffusivity and two with low diffusivity. They used mathematical algorithms to determine the size and patterning of blocks made from each material. The metamaterial achieves separation by cloaking one compound while concentrating the other.
“By designing the diffusivity of the metamaterials, we can make the atoms of one compound go one way, and the atoms of another compound go a different way. We are manipulating the physical properties to control the directions that atoms take through the metamaterial shell,” notes Maldovan.
The result is an anisotropic metamaterial — i.e., one whose structure favors flow in certain directions — that can direct a specific chemical around the shell or concentrate it within the shell (Figure 1).
Figure 1. Metamaterial guides component B to core, enabling more-effective separation from component A. Source: Georgia Institute of Technology.
“Our study has been initially performed on non-porous membranes (e.g., polymers) but we are planning to extend our approach to porous membranes,” Maldovan adds.
A recent article in Scientific Reports details how the metamaterial can separate a 50:50 mixture of nitrogen and oxygen by concentrating the oxygen.
The team now is collaborating with another researcher at Georgia Tech to fabricate a prototype separation device. “The idea is to have an initial sample by the end of the summer,” says Maldovan. “We will start the trials with oxygen and nitrogen, since we have already designed the polymeric matrix of the metamaterial to perform separation of these two gases. After success is achieved with O2 and N2, we will extend our research to other chemical compounds.”
“This is a new field and we will certainly encounter challenges and difficulties in the process of fabrication and testing. But we are expecting to have a functional experimental device fully characterized within the next two years.”
Then, the researchers intend to work on optimizing the separation efficiency of the device.