Higher-Selectivity Gas Separation Beckons
A novel computational method enables rapid design and development of an advanced filtration system for reducing greenhouse gas emissions, say its developers. The team, comprised of scientists from Columbia University, New York City, and the University of South Carolina (USC), Columbia, South Carolina, combined big data and machine learning (ML) to identify gas-filtering polymer membranes with markedly better selectivity for carbon dioxide. The approach has broader applicability, they believe.
“Our work points to a new way of materials design and we expect it to revolutionize the field,” enthuses Sanat Kumar, professor of chemical engineering at Columbia.
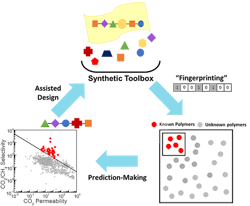
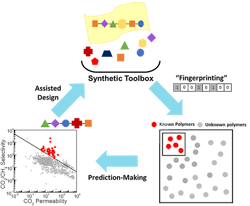
Kumar and his collaborators created a ML algorithm to investigate what structure would make the best membrane to separate CO2 from other gases. The algorithm correlates the chemical structure of the 1,000 currently tested polymers with their gas transport properties. The team then applied the algorithm to more than 10,000 known polymers to predict which would produce the best material in this context.
Starting with knowledge of the structure of the polymer building blocks, the team then developed a machine learning algorithm that finds the best material for a given application. Source: Columbia University.
The resulting 100 polymers had never been tested for gas transport but were predicted to surpass current membrane performance limits for CO2/CH4 separations. Science Advances contains more detail.
Next, to test the algorithm’s accuracy, Brian Benicewicz’s group at USC, synthesized two of the most promising polymer membranes and found they exceeded the upper bound for CO2/CH4 separation performance, exhibiting selectivities around 7 and 5.5 times better.
“This means if you contact a membrane with CO2/CH4, and let us say the selectivity is 10,…CO2 will go through in 10 times the volume as CH4. So, if you started with a 50/50 mixture then your outlet composition would be 90/10 CO2/CH4. Let us say our selectivity is 5 times — namely it is 50. In that case, the outlet would be 98/2 CO2/CH4, which means you do a better job of getting the CO2 out of the feed stream in that you lose less CH4,” elaborates Kumar.
The researchers say the method is easily extendable to other membrane materials. Thus, their next step is to generalize their approach to other material properties. “That is simply an exercise of inputting more data. Going beyond that is a question of inverse design. Namely, if you want a polymer (material) with a desired property, can we tell them which polymer to try? For the moment this is simply the opposite of what we currently do now — that is, if you give me the structure of the material, I can give you properties. In inverse design, you give me properties and I will find you some target materials that satisfy these goals,” says Kumar.
The methodology has significant potential for commercial use. “If we better purify streams, say, coming out of gas-fired power plants or of fracked gases, then this technology has implications in power plants, fracking of gases, CO2 sequestration, etc.,” believes Kumar.
The team also intends to explore membranes for water purification or other applications, but has not yet started this work.
“ML is a very powerful tool for design. It allows you to find new materials for a purpose, but at the current stage it does not help with understanding. Namely, it can find you better materials for a particular application. However, it will not tell you why this material is better,” adds Kumar.