Researchers Develop Water-based Electrochemical Method for Ammonia Synthesis
Researchers at the University of Bonn have developed a new method to produce ammonia using renewable electricity, water and nitrogen gas instead of methane-based hydrogen. The team’s work, published in Nature Communications, outlines a system that could provide an alternative to the energy- and carbon-intensive Haber-Bosch process, according to the press statement.
Ammonia, essential in fertilizer production, is typically synthesized using fossil fuels. The new method uses the lithium-mediated nitrogen reduction reaction (LiNRR), in which lithium reacts with nitrogen to form a compound that can then be converted into ammonia if hydrogen is available. According to the researchers, the system traditionally depends on alcohols or solvents as hydrogen sources, which limits its practicality and sustainability.
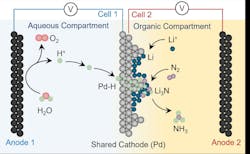
To address this, the team employed a palladium (Pd) foil that functions as both an electrode and a membrane. Palladium allows hydrogen atoms to pass through, enabling a setup where water-based electrolysis supplies hydrogen atoms to the reaction zone, separated from the water environment. This design eliminates the need for sacrificial hydrogen donors, the researchers said.
Experimental verification using deuterium-labeled water confirmed that the hydrogen used in ammonia formation came directly from water. According to the press statement, the study demonstrates the technical feasibility of a more sustainable ammonia synthesis process, although current yields remain far below levels needed for industrial application.
“We generally view this system as a model for the time being, as there are several practical difficulties,” said Nikolay Kornienko from the Institute of Inorganic Chemistry at the University of Bonn. Because high voltage is required to reduce lithium ions to metallic lithium, energy efficiency is limited to around 25%, according to press statement. The process must also be conducted in strictly air- and water-free environments due to the extreme reactivity of lithium metal. In addition, a porous solid electrolyte interphase (SEI) forms on the lithium layer, similar to lithium-ion batteries. This SEI must be permeable enough to allow both nitrogen gas and hydrogen to reach the reactive lithium surface, which is essential for the continued synthesis of ammonia, the team noted.
The researchers have filed a patent application for the system. Further development is needed to improve reaction rates, efficiency and selectivity before the process can be scaled for economic use.
“We are still in the early stages,” said the team. “In general, research needs to be done on the reaction rates and selectivity of the system.”
About the Author
Amanda Joshi
Managing Editor
Amanda Joshi has more than 18 years of experience in business-to-business publishing for both print and digital content. Before joining Chemical Processing, she worked with Manufacturing.net and Electrical Contracting Products. She’s a versatile, award-winning editor with experience in writing and editing technical content, executing marketing strategy, developing new products, attending industry events and developing customer relationships.
Amanda graduated from Northern Illinois University in 2001 with a B.A. in English and has been an English teacher. She lives in the Chicago suburbs with her husband and daughter, and their mini Aussiedoodle, Riley. In her rare spare time, she enjoys reading, tackling DIY projects, and horseback riding.