Charged Risk: Taming Static Electricity in Glass-Lined Reactors
Key Takeaways
- Static electricity in chemical reactors can be catastrophic - As shown in the 2007 Kansas incident, electrostatic discharge caused a tanker truck to explode, destroying an entire storage facility. This demonstrates how dangerous static buildup can be in chemical processes.
- Modern conductive glass technology solves the problem - New electrically conductive glass linings embed semiconductive ceramics that safely dissipate electrical charges while maintaining all the corrosion resistance benefits of traditional glass linings.
On a sunny summer day in 2007 near Wichita, Kansas, a tanker truck was offloading naphthalene into a stainless-steel tank at a solvent tank farm when the container spontaneously ignited, catching fire and exploding, shooting projectiles in the air. This led to the evacuation of thousands in a nearby community. While there were no casualties, the explosion destroyed the entire storage facility, luckily not causing any injuries or fatalities in the nearby community.
An investigation determined that electrostatic charge buildup had caused a spark that ignited a solvent-air mixer in the vapor space in the vessel receiving naphtha from the tanker truck.
The Science of Static: Understanding Electrical Discharge Risks
The lightning you see in the sky during a thunderstorm is static electric discharge between the positively charged ground and the negatively charged clouds above, with an electrical potential difference of millions of volts that overcomes the usually good dielectric insulating properties of the air that separates the two.
This produces trillions of watts of power in the span of a few microseconds. Static charge is caused by friction of one material against another. When a liquid flows through a pipe, static electricity is generated.
This also happens when a liquid falls through a vapor space or two solid pieces rub against one another. However, static is almost immediately dissipated where the two materials involved are electrically conductive. In this, there are two material characteristics that decide if static electricity will dissipate or buildup because of friction between two materials.
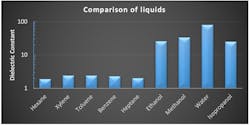
They include the dielectric constant (Figure 1) and the relaxation time:
Dielectric constant: A dielectric is an electrically poor or non-conductive substance in which the charge carriers are not free to move. A dielectric can be a gas, a liquid or a solid. In electrodynamics and electrostatics, dielectricity indicates the polarizability of a material by electric fields.
Relaxation time: In the natural sciences, relaxation refers to the transition of a system to a state of equilibrium via relaxation processes, often following an external disturbance. The relaxation time describes the characteristic time in which a system approaches the steady state, usually exponentially.
Static electricity is a constant risk in a chemical reactor because of the relative movement (friction) between the agitator/side walls/baffle and the reactants and the addition of solids and liquids into a reactor.
However, designing reactors to rapidly dissipate charge and prevent buildup can eliminate static discharge risks. Electrostatics must always be considered as a local phenomenon. Wherever higher speeds create friction, electrostatic charging can occur. Earthing via one component for the entire reactor may therefore not be sufficient. For instance, an electrostatic charge build-up on the liquid surface cannot be prevented via an earthed bottom outlet valve if there is a non-conductive liquid in between.
Static electricity is a constant risk in a chemical reactor because of the relative movement (friction) between the agitator/side walls/baffle and the reactants and the addition of solids and liquids into a reactor.
However, designing reactors to rapidly dissipate charge and prevent buildup can eliminate static discharge risks. Electrostatics must always be considered as a local phenomenon. Wherever higher speeds create friction, electrostatic charging can occur. Earthing via one component for the entire reactor may therefore not be sufficient. For instance, an electrostatic charge build-up on the liquid surface cannot be prevented via an earthed bottom outlet valve if there is a non-conductive liquid in between.
Glass-Lined Reactors: Where Chemistry Meets Vulnerability
Glass is an attractive construction material when corrosives are involved in a chemical reaction due to its non-corrosiveness, reasonable abrasion resistance and excellent release properties. Except for a few materials that attack it, borosilicate 3.3 glassware is extensively used in chemical laboratories around the world for experimentation and lab-scale production of all sorts of chemicals.
But borosilicate glass is inherently fragile, so it does not withstand positive pressures or tensile stress. Also, due to the production method used in borosilicate glassware, they are limited to 250 liters for a vessel or reactor. However, starting in the mid-1800s, the German manufacturing industry found ways to apply layers of a glass formulation as a slurry onto specially prepared carbon steel surfaces, with intermediate steps of high-temperature firing in electric or gas furnaces at approximately 1700° F. As it cools, the steel contracts far more than the glass layering, which results in the glass being under great compressive stress, and as a result, the bonded glass is extremely strong.
Since 1900, it has also been possible to glass-line large vessels, which were common in the beverage industry. The result is a six-layered composite material of bonded glass lining on a steel substrate that has nearly all the properties of glass, plus the strength of steel. This unique material has found widespread use in the chemical industry due to these qualities at a lower cost than exotic alloys, zirconium, tantalum and titanium.
Even so, glass has its own downsides: It is electrically nonconductive and has considerable resistance to heat flow (or has a very low U-value of coefficient of thermal conductivity). Each of these areas presents operational challenges depending on the process chemistry application. However, solutions do exist for glass-lined equipment to overcome these challenges.
Danger Zones: Mapping Static Buildup in Reactor Systems
The reactor consists of a jacketed vessel with a defined nozzle pattern, an agitator system of various geometries and configurations, and a baffle with one or more functionalities.
Each component plays a role in the performance of the overall reactor system and the efficiency of the process train for the chemical being produced.
The jacketed vessel is coated with a glass lining on the inside product contact surfaces, making it almost entirely corrosion resistant. However, it is also prone to static charge buildup and has reduced capacity for heat transfer. The vessel has a working volume or nominal volume and an unused volume, often referred to as the “vapor space.”
The agitator system is primarily designed for moving the vessel contents or reactant in a way that enhances their ability to react (mass transfer), and, as such, there may be one or more sets of blades that direct the flow axially, radially or a combination of both. The power input by the turbines, also called stirrers or agitators, into the process is a measure of dissipated power by the liquid depending on its density, viscosity, and turbine speed and diameter. There are different turbines for different processes or multipurpose reactors.
The baffle prevents the liquid contents of the vessel from moving en masse and creates the turbulence necessary for proper mixing. The baffle has various cross sections ranging from just a simple round over a flattened, round beaver tail to a delta. Also, a liquid surface baffle is available to support a better introduction of gas from the vapor space or floating solids. The baffle can also be designed to act as a heat exchanger (tube bundle), which will be described in the second part of this three-part series.
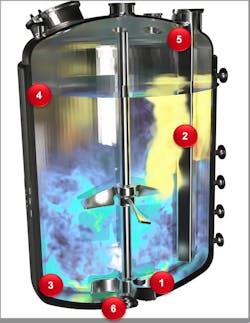
The areas inside a stirred reactor that are prone to friction and electrostatic charge buildup include (Figure 3):
- Tips of agitator blades
- The surface of the baffle
- Bottom brim
- Interface between the liquid level and the vessel wall
- Inlet of nozzles or baffles
- The bottom outlet nozzle from the reactor where the liquid swirls out under pressure
Conductive Innovations: Engineering Static-Safe Solutions
Traditionally, operators have reduced agitator speed or added ingredients to minimize electrostatic buildup risk. In practice, neither option is ideal due to process requirements for specific power input, product purity, and reaction chemistry.
Modern solutions avoid these compromises by addressing the root cause: each potential static buildup point in a glass-lined reactor can now be effectively grounded. For example, electrically conductive glass linings represent a significant advancement for pharmaceuticals and fine chemicals manufacturing.
These specialized materials embed electrically semiconductive ceramics within the glass matrix. Through precise application and high-temperature baking, technicians create a three-dimensional network of semiconductors that safely dissipate electrical charges without sacrificing other critical properties:
- Strong resistance to acid attack
- Enhanced resistance to alkali exposure compared to conventional glass linings
- No heavy metal content
- No catalytic effect
- Light blue coloration for improved process observation and simplified cleaning verification
- Compatibility with standard integrity testing protocols (spark test as per DIN EN ISO 28721)
These conductive glass systems have become standard solutions for pharmaceutical applications and API production. The technology is equally valuable for any process where electrostatic discharges pose risks, including during equipment cleaning operations. Recent innovations extend to outlet valve components, where conductive designs now incorporate FDA-approved and ADCF-certified materials.
Manufacturers have created systems particularly suitable for processing poorly conductive media and solvents by glass-lining valve cones and bodies with conductive formulations. Moreover, there are electrically conductive feed pipes, gaskets and temperature probes. These components can significantly enhance safety in areas where ignition hazards exist. Fire-safe valve designs complement these advances with short reactor nozzles (‘block flange’) that eliminate dead spaces and prevent product residue accumulation. With all product-contact components featuring electrical conductivity, these systems minimize spark generation risk while meeting rigorous certification standards.
Electrostatic charge buildup in industrial processes poses significant safety and efficiency concerns for chemical manufacturers worldwide. The advances in glass-lined equipment design (as well as related features such as feed-pipes and gaskets) offer solutions to manage risk and improve process performance and plant productivity while enhancing safety. ⊕
About the Author

Tom Patnaik
Vice President, Sales & Service
Tom Patnaik is a mechanical engineer with a bachelor's degree from National Institute of Technology (NIT), India, and a master's degree from Texas A&M University. He has over 25 years of experience in the chemical process equipment industry, focusing on micronization, reaction, filtration and vacuum drying. Currently, he serves as vice president of sales & service, Thaletec USA, responsible for raising awareness and acceptance of the company's glass-lined reactors, heat exchangers and columns, among others.

Dr. Ing. Christian Stentzel
Head of Research & Development
Dr. Ing. Christian Stentzel is a mechanical engineer from the Technical University in Dresden, Germany, with over a decade of experience in various research capacities. He joined Thaletec GmbH in 2021 and currently serves as the head of research & development, responsible for bringing innovative solutions for the company's glass-lined product lines.