Lower-Cost Route to Acrylic Acid Nears
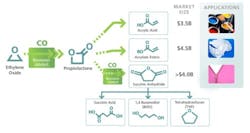
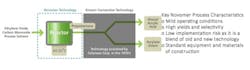
Novomer recently received a $5-million grant from the Clean Energy Manufacturing Initiative of the U.S. Department of Energy (DOE) to aid its efforts in developing a new process to make acrylic acid. Instead of relying on propylene, the typical feedstock, the new route uses ethylene oxide, thereby leveraging cost-advantaged North American shale gas. The Waltham, Mass.-based company's two-stage process can produce chemicals such as acrylic acid, butanediol, tetrahydrofuran and succinic acid (Figure 1). Products made via Novomer's technology are expected to be 20–40% lower in cost, partly because of the use of lower-cost starting materials compared to current production processes.
Initial efforts will focus on acrylic acid, a 4.4-million-metric-ton/year global petrochemical business worth around $7 billion in 2011. Global demand for crude acrylic acid should increase at a rate of 4.8% annually from 2010 to 2015, according to a 2011 forecast by IHS Chemical. This strong growth stems from rising demand for super-absorbent polymers, adhesives and sealants. Moreover, increase in construction and building activities in emerging economies should keep driving acrylic-acid demand in the coming years.
TWO-STAGE PROCESS
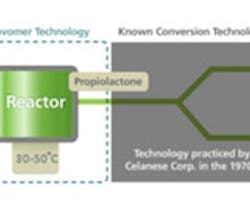
In the first stage of the process, a novel catalyst spurs the reaction of carbon monoxide and shale-gas-based ethylene oxide to form propiolactone. This then is converted into acrylic acid in the second stage using established technology.
The core catalyst that enables the technology is a homogeneous cobalt-based compound first developed at Cornell University, Ithaca, N.Y., by Professor Geoffrey Coates and further optimized by Novomer. This catalyst is 99% selective, so almost no raw materials are wasted in the process. Moreover, it functions at only 30–50°C, compared to current acrylic-acid processing catalysts that typically operate at 200–250°C. The combination of high selectivity and moderate temperature mean that Novomer's process will have a lower carbon and energy footprint than the propylene oxidation process to make acrylic acid.
Figure 1. New process can make a variety of chemicals that have large, established markets.
Figure 2. Process couples new catalytic process for producing propiolactone with established technology to convert that intermediate.
MIKE SLOWIK is director of chemicals for Novomer, Inc., Waltham, Mass. HARSHAL SAWANT is a business development associate for Novomer. E-mail them at [email protected] and [email protected].