Fluidization Foils Electrocatalyst Fatigue
Fluidizing catalyst particles in electrolyte instead of attaching them to electrodes provides a more efficient and stable method to conduct electrocatalytic reactions, say researchers at Northwestern University, Evanston, Ill. The approach could improve production processes for electrolysis and electrochemical energy conversion and storage, they add.
With the conventional approach of catalysts attached to electrodes, voltage continuously applied through the electrode causes electrochemical stress on the material. Over time, their catalytic performance can decay due to accumulated structural damage in the electrode as a whole or on individual particles.
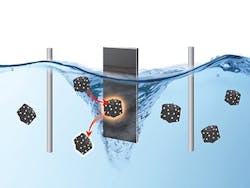
The team’s approach avoids the continuous stress by fluidizing the particles in the electrolyte. The rotating particles experience electrochemical stress only momentarily when colliding with the electrode. Collectively, the output from the individual collision events merge into a continuous and stable electrochemical current.
Figure 1. Catalytic particles work in rotation and become momentarily “electrified” when they collide with the electrode, leading to improved fatigue resistance. Source: CCS Chemistry/Northwestern University.
“Fluidized electrocatalysis breaks the spatial and temporal continuum of electrochemical reactions, making the catalysts more efficient,” says Jiaxing Huang, professor of materials science and engineering at the university’s McCormick School of Engineering, who led the research. “Fluidization also reduces the mass transport limit of the reactants to the catalyst, since the particles are swimming in the electrolyte.”
Using a commercially available Pt/C catalyst, which is susceptible to severe performance decay, to catalyze oxygen evolution, hydrogen evolution and methanol oxidation reactions, the team found their fluidization method resulted in higher efficiency and stability. Their findings appear in the journal CCS Chemistry.
Huang believes his method could be applied to a variety of different types of materials and drastically extend runtime for low-cost, more-abundant but otherwise unstable electrocatalysts.
“Fluidized electrocatalysis basically collects a lot of transient current from particle-electrode collision to yield a continuous overall current output, which is generic to all reactions using particle catalyst. We found they’re particularly useful for reactions suffering from unstable catalysts such as Pt and MOF [metal organic framework] materials for oxygen reduction reactions, or reactions where the electrode kinetics is limited by inefficient mass transport, or those involving gas evolution that may cause catalysts pulverization and detachment from the electrode surface, such as hydrogen evolution reactions and oxygen evolution reaction,” he explains.
“Such materials tend to aggregate and sinter when exposed to continuous and long-term electrochemical voltage. Such fatigue problems should be well addressed by fluidizing the corresponding catalytic particles,” he adds. The team hasn’t yet completed this project, but is looking into these reactions.
“This work stands to bring a new concept and provide three model reactions for proof-of-concept demonstration. The three model reactions already represent most common mechanisms of catalyst fatigue. We need funding to continue to explore other systems,” he adds.
However, the approach may encounter volume-related constraints in volume-sensitive applications. “This refers to the need for fluidized reactions to have an electrochemical cell allowing electrolytes to flow, regardless of how small it is. The need for flowing and liquid electrolytes limits the lower end of the reactor sizes, in comparison to other systems using solid state electrolyte or just a very thin layer of electrolyte gels,” notes Huang.
While the team used magnetic stirrers to achieve fluidization in the lab, Huang points out there are many ways to induce flow on an industrial scale, including by a pump and gravity, or by rotating the electrochemical cell or a rotating electrode to stir up the reactions.
“I hope other researchers consider our method to re-evaluate their catalysts. It would be exciting to see previously deemed unusable catalysts become usable. We welcome suggestions and input about catalyst fatigue problems in your electrochemical reactions. This helps us to identify new problems to address,” concludes Huang.