Green Hydrogen Looms
A highly effective electrochemical process for ammonia-to-hydrogen conversion is a significant step towards widespread, environmentally friendly hydrogen fuel cell production, believe researchers from Northwestern University, Evanston, Ill., and SAFCell, Inc., Pasadena, Calif. The ammonia would serve as a carrier for hydrogen delivery, they note.
“The bane for hydrogen fuel cells has been the lack of delivery infrastructure,” says Sossina Haile, professor of material science and engineering at Northwestern’s McCormick School of Engineering. “It’s difficult and expensive to transport hydrogen, but an extensive ammonia delivery system already exists... If you give us ammonia, the electrochemical systems we developed can convert that ammonia to fuel-cell-ready, clean hydrogen on-site at any scale.”
Their process requires a lower temperature than that needed in traditional thermal ammonia-to-hydrogen routes, 250°C versus 500–600°C, and could use renewable electricity, the researchers add.
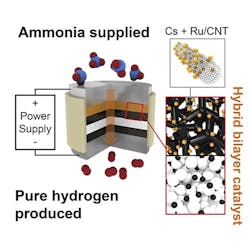
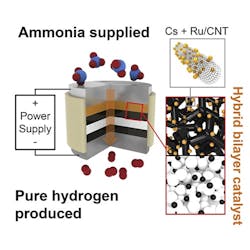
An electrochemical cell with a proton-conducting membrane and integrated ammonia-splitting catalyst drives the conversion.
“The ammonia first encounters the catalyst that splits it into nitrogen and hydrogen,” explains Haile. “That hydrogen gets immediately converted into protons, which are then electrically driven across the proton-conducting membrane in our electrochemical cell. By continually pulling off the hydrogen, we drive the reaction to go further than it would otherwise.”
The pure hydrogen generated doesn’t need separation from any unreacted ammonia or other products, and can be directly pressurized for high-density storage by ramping up the electrical power, the researchers note, adding that the device’s electrical current directly produces hydrogen, with no loss to parasitic reactions. The journal Joule contains more details.
Figure 1. Zero ammonia crossover and no side reactions result in pure hydrogen product. Source: Reprinted from Lim et al., Joule 4 1-10 (2020).
In addition, the cells’ metal and polymer components and solid-state electrolyte make them very mechanically, thermally and chemically robust. “Because cells/stacks run at 250°C, they are very tolerant to typical impurities found in ‘fuel streams.’ These include sulfur components, carbon monoxide and carbon dioxide, most hydrocarbons, and obviously ammonia,” says Calum Chisholm, CEO and president of SAFCell. “We have been running our cells and stacks on industrial grade ammonia and have seen no adverse effects up to 5,000 h of operation,” he adds.
The team, which has previously focused on electricity production from hydrogen fuel, will next explore methods to produce ammonia in an environmentally friendly way.
“The solid acid technology has potential to produce ammonia from ‘green’ hydrogen (i.e., hydrogen from renewable sources) and nitrogen from the air in a very simple and economical way. Sossina is currently beginning to explore this option and SAFCell would be more than happy to scale up her successful research,” notes Chisholm.
To implement the technology demonstrated by the team for hydrogen production, the stack must be scaled up at least 100× from its present size (1–2 kg H2/day) to meet the quantity of hydrogen needed at refueling stations (400–2,000 kg H2/day).
“It is possible that five, 400-kg-H2/day stacks could be used in parallel for initial 2,000 kg H2/day systems, but CAPEX [capital] and OPEX [operational] costs will be decreased by using one bigger stack,” notes Chisholm. “Whenever you scale up this much, there are always manufacturing and operational ‘details’ that need to be sorted out. We do not see any major roadblocks, but without a doubt it will take a lot of work,” he adds.
The team performed a detailed efficiency analysis based on data taken at SAFCell on scaled-up cells and stacks. “A large system that would be applicable for FCEV [fuel cell electric vehicle] refueling stations, would be around 88% efficient,” he says.
Some leading firms are looking into use of ammonia as a liquid hydrogen carrier, with desire to use our scaled-up systems, concludes Chisholm.
“Cracking the ammonia and then separating the produced hydrogen from the nitrogen, and then further compressing the produced hydrogen all in effectively one step does allow for a dramatic reduction in system complexity and cost,” says Chisholm. “You get an efficiency boost from using the waste heat produced by ohmic heating losses inside stack to drive the endothermic ammonia cracking reaction,” he adds.