Carbon Dioxide Conversion Gets New Option
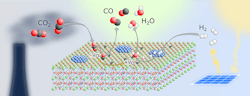
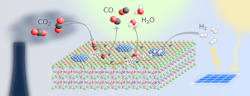
A stable, relatively cheap perovskite catalyst (Figure 1) opens up a novel route to convert carbon dioxide (CO2) into useful substances such as methanol, other chemical base materials and synthetic fuels, claim its developers at the University of Vienna, Austria.
Researchers led by Christoph Rameshan at the Institute of Materials Chemistry of the university have focused on the reverse water-gas shift (rWGS) reaction that converts CO2 and hydrogen into water and carbon monoxide — with the latter capable of further processing.
“We tried out a few things and finally came up with a perovskite made of cobalt, iron, calcium and neodymium that has excellent properties,” he says.
The host lattice of the perovskite itself is active for the WGS and rWGS reactions. The key to the high activity achieved is doping it with cobalt, which is easily exsolved under rWGS conditions. Importantly for the rate of catalytic reaction, the nanoparticles formed by exsolution are finely dispersed across the surface and not prone to sintering.
“The advantages easily compensate for the slightly more complex synthesis route. To really quantify the benefits, we would need additional testing in a pilot plant, which we are currently preparing,” notes Rameshan.
Figure 1. Material offers a tunable host lattice for efficient CO2 adsorption. Source: Applied Catalysis B: Environmental, 5 September 2021.
His team now is trying to improve the reactivity of the catalyst, including by tuning the exsolution properties to control the temperature at which nanoparticles form during the rWGS reaction. This could boost catalyst performance by a factor of 10–50 times, Rameshan believes. He also has found a replacement for the expensive neodymium currently used and is actively searching for new dopants.
To be economically feasible, the reaction would need coupling to a large source of CO2 such as a power or chemical plant, he reckons.
“From an engineering point of view, all the technology is available. The two most crucial parts are: on the one side, the supply of the required hydrogen — that can be produced ideally by electrolysis — as this is a cost-intense factor; and the production of our catalyst on an industrial scale is still missing. For this, we just started a cooperation with a chemical engineering department to go to the next scale with a lab-scale demo plant.”
Rameshan already has contacted industrial companies and is confident this will lead to future cooperation and funding.