High-Throughput Experimentation Qualifies Commercial Catalyst
Maximizing process efficiency to ensure competitiveness and improved margins continuously drives large-scale production of industrial chemicals. The catalyst is a key component of many of these processes. It not only requires tuning to achieve maximum activity but also tailoring of its physical and chemical properties to the surrounding process. High product selectivity avoids wasting valuable feedstock and protects the downstream section from energy-intensive separation and pollution of the equipment with side products. Furthermore, the catalyst must withstand feed impurities and provide flexibility with regard to process conditions.
Upgrading a process during a turnaround with the latest-generation of the catalyst or even switching to an alternative technology offers significant opportunities to increase plant profitability but also involves technical and economic risks. This article describes how high-throughput catalyst performance evaluation can support chemical production plants in quantifying the potential gains and mitigating the risks early on in the catalyst selection process.
Sipchem, Al Khobar, Saudi Arabia, a globally recognized manufacturer of base chemicals, intermediates and polymers, cooperated with hte, an independent catalyst tester based in Heidelberg, Germany, to evaluate commercial hydrogenation catalysts for the gas-phase conversion of dimethyl maleate (DMM) to butanediol (BDO). Performance results of the laboratory-scale test were in line with the commercial-scale reference plant, and stability data supported Sipchem’s decision for catalyst replacement.
Parallel Testing Program
Continuous gas-phase hydrogenations of high-boiling substrates often operate under a great excess of hydrogen, with molar H2:substrate ratios typically exceeding 50:1. Examples include DMM to BDO, fatty acid methyl esters to fatty alcohols, aldehydes to oxo alcohols, and nitro aromatics to aromatic amines. Such hydrogenations require enormous hydrogen flows and represent a challenge at both commercial and laboratory scale. Commercial catalyst design must provide low pressure drop and high mechanical strength to deal with the high flow rates. Furthermore, the design of the internal pore structure must prevent capillary condensation [1,2].
The commercial catalyst system tested here was a stacked bed with a guard layer (top) and a conversion layer (bottom). hte developed a customized packing protocol to ensure defined and reproducible reactor filling; hte can handle shaped materials, fibers or powders within high-throughput setups [3].
The catalyst was crushed and sieved to a certain particle fraction and physically mixed with an inert to achieve a plug-flow pattern and avoid channeling. This allowed control of the pressure drop and fostered isothermal operation by uniform heat distribution along the catalyst bed in stainless steel reactors with an inner diameter of 5 mm and a length of 400 mm.
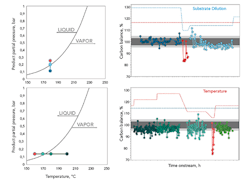
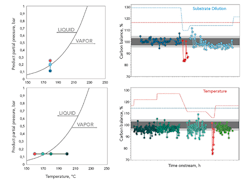
Figure 1. Graphs show carbon balance for different feed dilution ratios and reactor temperatures in comparison to the phase diagram of butanediol as the high-boiling product.
hte supported Sipchem’s experimental program and the objectives of the study to test the impact of process conditions while still considering individual catalyst effects. The sophisticated reactor filling concept enables conducting several experiments in parallel. Here, these involved 1) residence times variation (achieved by loading different catalyst masses); 2) testing of spatial catalyst arrangements; 3) changes in the mass ratio of guard to conversion bed; and 4) duplicate positions to verify the rig performance and data quality. hte uses a fully integrated digital laboratory workflow based on proprietary software. Its myhte relational database combines all available catalyst and reaction data to allow advanced structure performance evaluations.
hte used a 16-fold high-throughput gas-phase system for this project. It serves reactors with inner diameters of 4 mm to 15 mm to test materials from powder scale-up to commercial shapes. Mass flow controllers and syringe pumps provide precise dosage of gas feeds and liquid components, respectively. Each of the 16 reactors received the same amount of hydrogen and methyl ester feed. A block heater controlled the temperature and furnished a uniform 15-cm-long isothermal zone (with a maximum temperature deviation of ±1K). Nitrogen dilution of the reactor effluent reduced partial pressure of high-boiling products to avoid condensation. Safety condensers trapped heavy product components. The challenge posed by high volume flow was overcome by adapting the downstream assembly to reduce the pressure drop across the entire unit. A PolyArc gas-chromatograph (GC)/flame-ionization-detector system analyzed the product stream for enhanced oxygenate detection.
Results
The experimental design exposed the 16 reactors to 16 different process conditions consecutively. This combination resulted in 256 experiments with more than 1,280 data points; these were screened within only 22 days during April and May 2020. As we will discuss, activity and selectivity profiles as well as decay data from these high-throughput experiments can support catalyst performance evaluation.
The combination of hot-gas flow meter and GC analysis data with a detailed assignment of all product species allows the closing of material (carbon, hydrogen) and flow balances across the entire rig.
The multistep hydrogenation of DMM to BDO is an exothermal reaction that proceeds with volume contraction. High pressure and low temperature thermodynamically favor high product yields. However, the desired parameter space resides fairly close to the dew point line of the main product and needs improved catalyst design on the one hand but very precise process control on the other hand to avoid condensation inside the pores of the catalyst.
Figure 1 shows how temperature and feed dilution were decreased until the dew point line was touched to verify the catalyst performance at this desired sweet spot. Precise control of temperature and partial pressure allowed the recording of data close to phase transition. Fluctuation of the mass balance increased near the expected condensation point of BDO. Beyond this critical condition, the carbon balance dropped significantly and liquids were recovered in the safety condenser. The results do not suggest a superimposed condensation based on catalyst pore effects, which is a desirable ability for these kinds of catalysts.
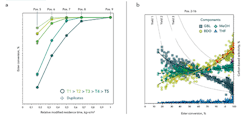
Facile filtering and clustering of the bulk data supported by the advanced data-handling capabilities of myhte gave quick access to the relevant correlations. For example, Figure 2a shows the conversion-versus-residence-time chart; it only considers reactor positions with equal guard-to-conversion-bed mass ratios.
Figure 2. Graph (a) shows conversion versus modified residence time for selected reactors with constant guard-to-conversion-bed ratio at different reactor temperatures, while graph (b) plots carbon-based product selectivity versus ester conversion of bulk data with highlighted experiments at different reactor temperatures (red circles), and spatial catalyst arrangement (stars).
The catalyst exhibits excellent activity. Full conversion was achieved, even when operating far below a residence time of 500 kg×s/m3. Supply of extremely high flows by the rig decreased the residence time toward an area of differential conversion. Elevating the temperature increased ester conversion significantly. Raising partial pressure of the substrate had a similar effect. Duplicate positions, shown by cross symbols, reveal very good reproducibility of conversion and selectivity (not shown) over the entire conversion range.
Reaction network and performance analyses were carried out using superposition plots that allow for a tailored filtering of individual results within different layers. The background of Figure 2b shows the bulk data split into the selectivity of the main components versus ester conversion of all experiments. The dashed lines are provided only to better illustrate the selectivity curves. The target reaction is a multistage hydrogenation. DMM is converted to gamma butyrolactone (GBL) as an intermediate (squares) within the initial step, splitting off two methanol (MeOH) molecules as a couple product (diamonds) that will not be converted further. This can be shown by extrapolating the trajectory of the selectivities toward zero conversion. GBL and MeOH selectivity end up between 0 and 1. The selectivity of the BDO (circles) as the target product is zero at the initial step, suggesting it is formed consecutively from GBL. Running high conversion leads to dehydration either of GBL or BDO to form tetrahydrofuran (THF) (triangles).
A closer analysis of the product distribution revealed the formation of side products starting from both ester and the diol. Accordingly, feedstock losses will occur due to a parallel and consecutive side reaction. The reaction particularly is prone to forming polymeric products if operated at very high temperature and residence time. Therefore, reactor design and choice of operating parameters should take this into account.
However, within the tested parameter space, the side product yield was negligibly low, showing a good ability for selective diol production of the given catalyst. The overall yield (by carbon) of GBL, BDO and THF, which represent high-value commercial products, exceeded 70%.
In Figure 2b, yellow to red circles show the influence of reaction temperature on the BDO selectivity. In between, an ester conversion of 0–100% selectivity is independent of temperature. Running toward full ester conversion, the BDO yields meet the calculated values for thermodynamic equilibrium. At the highest temperature, a significant drop of the diol selectivity indicates the shift toward formation of the consecutive product (triangles) as well as increased side product formation.
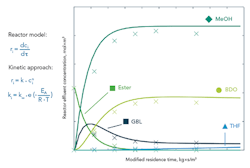
Figure 3. Model data (lines) quite well match measured results (crosses) for reactor effluent concentration versus modified residence time.
Although the purpose of the guard bed (yellow star, Figure 2b) is to protect the downflow catalyst against impurities or initial side products, its target product selectivity is high and comparable to the main conversion bed (green star, Figure 2b). Accordingly, the commercial reactor will waste no space with a lower-performing layer. Purple star symbols indicate different catalyst ratios. High guard bed contents (dark symbols) and low guard bed contents (lighter-colored symbols) show product yields at the same level. This enables processing at a low content of top guard layer, which, in turn, significantly reduces the effort needed during catalyst top-layer skimming. Within the entire screening, the catalyst system showed good stability. The tested catalyst combines high activity and selectivity, leading to a superior space/time yield.
Fitting a formal kinetic approach to the experimental data enabled determining a preliminary mathematical description (Figure 3). It assumed isothermal conditions and plug-flow behavior, and neglected mass-transfer limitations. Hydrogen had been fed in excess and, therefore, is not considered within the equations. The balance for an ideal plug-flow reactor and power law approach were used as model equations. All data were fitted simultaneously to obtain the activation energy, frequency factor and reaction order of each single reaction pathway.
The calculated data are in good agreement with the screening results and allow for a prediction of effluent concentration as a function of temperature and residence time. Kinetic data models can support the experimental design during the implementation of new catalyst product lines and reduce downtime. This approach shows how high-throughput units can assist in determining precise kinetic data setups.
Follow Up
The process was successfully transferred from commercial to laboratory scale; the application of high-throughput experimentation allowed for fast and efficient screening of activity, selectivity and decay behavior. Results agreed very well with the data from the commercial plant.
Based on hte’s results, Sipchem intends to trial the catalyst at the commercial unit’s next catalyst turnaround, which should occur before the end of this year. The results predict a longer runtime and overall yield enhancement of between 20% and 40% depending on the plant conditions. These benefits are of great value because they significantly raise the margin of the plant.
Sipchem and hte intend to work together on future projects. The current study provided a good understanding of the behavior of crushed catalyst processed with model feeds. Further work could involve testing shaped catalysts and processing real feedstocks.
ENRICO LORENZ is segment lead, petrochemicals, for hte, Heidelberg, Germany. TOBIAS ZIMMERMANN is segment lead, intermediates, chemicals and specialties, for hte. ALEXANDER HIGELIN is senior business development manager for hte. MORITZ DAHLINGER is a project coordinator for hte. JOACHIM HAERTLÉ is a project coordinator for hte. NILENINDRAN S. GOVENDER is an Al Khobar, Saudia Arabia-based senior staff researcher for Sipchem. SACHIN TELI is an Al Jubail, Saudi Arabia-based production engineer for Sipchem. ABDULAZIZ A. ALMATHAMI retired from Sipchem and now is an adjunct professor in the Dept. of Mechanical Engineering at Prince Mohammed Bin Fahd University, Al Khobar, Saudi Arabia. Email them at [email protected], [email protected], [email protected], [email protected], [email protected], [email protected], [email protected] and [email protected].
REFERENCES
1. C. Ohlinger and B. Kraushaar-Czarnetzki, “Improved processing stability in the hydrogenation of dimethyl maleate to gamma-butyrolactone, 1,4-butanediol and tetrahydrofuran,” p. 1,453, Vol. 58, Chem. Eng. Sci. (2003).
2. F. Càrdenas-Lizana, S. Gómez-Quero, C. J. Baddeley and M. A. Keane, “Tunable gas phase hydrogenation of m-dinitrobenzene over alumina supported Au and Au–Ni,,” p. 155, Vol. 387, Appl. Catal. A: Gen. (2010).
3. B. Mutz, P. Kolb, A. Higelin and D. Lenz, “Accelerated catalyst screening and scale-up for aromatics alkylation,” p. 51, Pet. Tech. Quart., Q1 (2021).