Spot problems with adsorbents
Always assess the consequences of system additions. While sometimes this may involve detailed calculations, often just paying attention to the laws of physics can head off problems.
One plant learned this lesson on a tank vent line for volatile organic vapor capture. Originally, the tank vented to atmosphere. The organics in the tank had approximately 12 psia vapor pressure. During normal operation, tank breathing losses amounted to a significant environmental and safety issue.
So, carbon canisters were put on the tank to reduce the breathing loss and a nitrogen purge was added. Upstream process upsets could occasionally get air into the tank but the continuous nitrogen purge prevented an explosive mixture from forming.
Initially the system seemed to work well. Sniff testing of the vent downstream of the carbon canisters indicated good performance. The canisters had to be changed once or twice every year. All project objectives were met.
However, a follow-up review aimed at reducing nitrogen consumption identified an inconsistency. For the nitrogen consumption rate with the entrained organic rate expected, the carbon canister life would be less than one day. (Evidently the likely life of the canisters wasn’t checked during initial size selection.) So, the plant started to delve into why the actual canister life was so much longer than expected. It found that the reason was that most of the organic vapors didn’t reach the canisters.
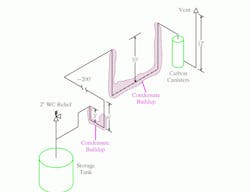
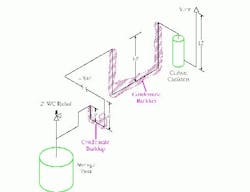
Figure 1 shows the field-confirmed tank and vent line configuration. The tank was protected from over-pressure by a vent relief valve set at 2 in. of water column. The calculated pressure drop in the line from the tank to the carbon canisters was less than 0.5 in. of water column. The line followed existing pipe racks. Because of this, the line from the tank to the carbon canisters included two large low spots.
Figure 1. Two large low spots in the line led to significant condensate buildup that blocked vapor flow. (Click to enlarge)
Over time, the low spots filled. (A quick check in the field by opening low-point drains in the vent pipe confirmed that both spots were full of liquid.) Once the buildup was sufficient to impose more than 2 in. of water column back-pressure on the tank, its safety relief valve opened. The nitrogen purge plus the organics left the tank via the safety relief. No flow went down the line to the carbon canisters. However, day-to-night temperature cycles allowed vapor at times to pass through both low points to the canisters.
Finally, enough daily cycles would saturate a canister, necessitating its replacement.
A completely different system for volatile organics was put in place. The new system used a small cooler in the vent line above the tank to condense the largest part of the organic load. A second step was the elimination of dead legs in the piping to the carbon canisters.
The initial project failed because it violated two laws of physics. First, material that vaporizes at one ambient temperature will condense when ambient temperature slightly drops. Second, flow goes to the low-pressure-drop route — in this case the pressure relief valve.
If your design modification violates the laws of physics, it won’t work either.
By Andrew Sloley, contributing editor, [email protected], and Bruce Veale, VECO USA, [email protected].