Since 1978, I’ve been designing processes, when I helped develop a successful new pharmaceutical route. Later, I spent five years in a pilot plant which ran more than 100 fermenters. Throughout this time, I have had to grapple with steam sterilization issues and have found that while the principles of steam sterilization are well known, designing actual systems can pose big challenges. Following are some do’s and don’ts gleaned from these experiences, as well as the challenges I still encounter in designing steam-sterilizing systems.
Steam sterilization should ensure that organisms do not contaminate plant equipment and products. Unfortunately, design miscues can compromise performance.
The effectiveness of sterilization is measured in Fo. [Each Fo represents conditions providing a 90% kill or a one log reduction of normal (D = 1.0) Geobacillus stearothermophilus (also known as Bacillus stearothermophilus) spores. Geobacillus stearothermophilus forms heat resistant spores that are used as a standard for moist-heat sterilization; very heat resistant strains with D = 2 are now available.] For a background bioburden of 1 million Geobacillus stearothermophilus spores, 15 Fo’s would ensure that there was only one-in-a-billion chance of a viable spore surviving.
The do’s
The following seven factors are crucial for successful sterilization.
1. Eliminate air from the system. Effective moist-heat sterilization depends on having saturated steam conditions. When air is present in the system to be sterilized, saturated steam temperature and pressure conditions no longer apply.
The air dilutes the steam and a lower temperature than expected is imposed at a given pressure. Trapped pockets of pure air completely prevent steam contact and provide dry-heat sterilization conditions. With saturated steam, one log of kill occurs at 121.1°C for one minute. Lower temperature necessitates much longer times to achieve each Fo. Dry-heat sterilization conditions require much higher temperatures and greater time to create one log of kill.
Two methods often are used for eliminating air:
Pre-vacuum cycles. Autoclaves commonly rely on a vacuum pump to eliminate air from the piping, tubing, equipment, clothing, gauze packing and chamber prior to introducing steam. A cycle of vacuum pull followed by a steam charge is often called a “prevac.” A series of three prevacs usually suffices to remove air, as long as there are no major leaks. The advantage of prevac cycles is that they can remove air from more-porous materials and potentially deeper “deadlegs.” The disadvantage is that a vacuum pump has to be purchased and maintained.
Steam turbulence and flow. When nothing is present that will trap air, vacuum cycles are unnecessary. Many autoclave loads and most fermenters and piping systems can be sterilized using a “gravity cycle.” This method depends upon steam flowing throughout the system in a directional way to ensure air is swept out of the system. Steam flows from points of introduction to steam traps, orifices or through valves that allow the air, condensate and steam to leave the system. The piping is designed with minimal pockets or deadlegs to trap air.
One technique to eliminate air from a stagnant area is to introduce steam in a way that disrupts the air and then sweeps it out of the system. The most common place to trap air is at a high point that is not vented through a trap or orifice. In fermentation, for instance, it is important to ensure the nozzles at the top of the tank, where the agitator seal and other ports exist, do not trap air. Sending steam in through the nozzle is one method to eliminate air. Designing so that steam flows out through a nozzle to a trap is another. A third method is to introduce steam from another port and point it toward the nozzle suspected of trapping air. The steam will create turbulence and disperse the air. Steam flow through the rest of the system (if designed correctly) will carry the air out.
Systems that require sterilization can become pretty complex, especially when piping systems must be included. So, during design, visualizing steam flow and sequencing automatic valves to direct steam flow in defined flow patterns is an important check. Also, choose valves, such as diaphragm ones, that do not have pockets that trap air.
The advantage of using steam turbulence and flow is that no vacuum pump is needed. The disadvantage is that more design skill is required to ensure proper flow of steam.
2. Place traps or orifices at low points to drain condensate and slope lines to them. All true low points in a system to be sterilized either should have a steam trap or an orifice to bleed condensate. If not, steam will cool, become condensate that will fall below the required sterilization temperature and thus fail to sterilize the system. The key is to recognize all low points and design in traps or orifices. Also, sloping lines toward the low points allows minimum buildup of condensate and allows it to drain.
When using saturated steam, condensate is unavoidable, because it is formed whenever steam condenses to heat an object or surface. The condensate, if it remains at the desired temperature for the desired time, will be sterile. When maintaining the temperature of condensate in a low line poses difficulties, a solution may be to raise the incoming steam pressure to the system and hence the temperature of the low line. The pressure of the steam, the rate at which steam and condensate flow through the orifice or trap, the size and slope of the low line, the amount of insulation and the volume of condensate to be eliminated all play a role in whether that low spot can maintain a given sterilization temperature.
3. Place temperature indicators at low spots and key points. Temperature indicators, usually permanently placed RTDs, provide the process engineer with a way of determining, both in real-time and for after-the-fact troubleshooting, if the system is properly sterilizing during routine production. Traps can fail, valves may remain closed and orifices can plug. Hence, a system that sterilized yesterday could fail today. Placing temperature indicators at each of the low spots where an orifice or trap exists enables detection and automatic alarming when temperature conditions aren’t met. Adding more instruments boosts costs, but also increases sterility assurance. Temperature elements permanently placed in other key places — at the steam source, in the vessel being sterilized or in a location that is a concern — also assist in monitoring the sterilization process.
4. Create as leak-free a system as possible. All systems should undergo a leak test prior to and after sterilization. Leaks are bad for several reasons:
• Pressurized air can leak into the system and void the saturated steam conditions, or formation of a slight vacuum during cool down can bring in organisms after sterilization.
• Also, during the fermentation, the leak may fill with media, thus providing a stagnant “fuse” through which foreign organisms can grow into the fermenter.
• Steam and condensate can leak out of the system and cause safety hazards.
The last system I designed had a computer-aided three-stage leak test performed after the steam sterilization phase. The test ensured leaks into and out of the system were minimized and also that key valves would not leak through when closed, to eliminate risk to the product that would be contained in the system.
Leaks occur. No leak is good, but not all leaks cause contamination. Leaks create a higher chance of contamination or decrease sterility assurance. My experience in the fermentation pilot plant showed that all leaks are not created equal when it comes to contamination. When sterile air was carried into a fermenter from an outside source and a leak was detected in the air line, a high rate of contamination occurred. I suspect a venturi effect caused non-sterile air from the outside to be pulled into the fermenter, contaminating it. However, an outward leak from a nozzle at the top of the tank caused a low rate of contamination.
5. Post-sterilization, follow steam with sterile air and maintain a positive pressure. After the steam sterilization cycle is complete, introduce sterile air into the system. If this is not done, when the steam flow is stopped, the steam will condense and begin to create a vacuum in the system. This vacuum may pull in non-sterile air from outside via a leak, resulting in a potentially high occurrence of contamination. Once air pressure is present in the system (I recommend 3 to 5 psig), maintain that pressure. It serves as a sterility assurance measure, increasing the likelihood that, if a leak occurs, the flow will be outward, not inward.
6. Design the system to be easily cleaned. In pharmaceutical applications, this is a pretty obvious statement, because the product needs to be kept clean. When the work is done manually, which often is the case for fermenters, improper cleaning can create steam sterilization problems:
• Layers of debris, media or product can make heating slower than expected or keep steam from penetrating to sterilize the material or system.
• Debris can cause steam traps or orifices used for sterilization to not work properly.
7. Look for unexpected temperature variations. A large article could be written on just this subject; here we can only describe the basics.
When sterilization of the system is to be validated, myriad thermocouples are placed in the system in conjunction with the permanent temperature elements to study the sterilization process. If two properly calibrated temperature elements in the same part of the system vary from one another significantly, then air, superheated steam or excess condensate probably is present.
Always suspect and verify temperature element calibration first. Once temperature elements are confirmed to be accurately calibrated if:
• Lower than sterilization temperatures are seen in low spots, suspect condensate problems.
• Lower than sterilization temperatures are seen in high spots, suspect air problems.
• Temperatures are too high for the pressure present, then suspect superheated dry-steam problems.
Unfortunately, when too-low temperatures are observed, it is not always possible to tell the difference between trapped air and excess condensate. In the case of condensate, as long as it meets temperature and time requirements, sterilization will occur. However, if air causes the temperature variation, then assume sterilization will not occur. Developing the ability to detect temperature variations is important and takes experience. Other devices can detect that air is present in a system, but temperature variation with thermocouples can actually help locate the source of the problem.
Hard-and-fast rules on how much temperature variation indicates a sterilization problem have not been provided, but the concepts presented apply. Consider the actual temperature elements used, with their given accuracies, when creating a standard for determining when temperature variation indicates a sterilization problem.
The don’ts
Let’s now look at five common mistakes.
1. Don’t create deadlegs. When designing piping for steam sterilization, it is all too easy to create areas where air pockets form and, thus, steam does not fully penetrate and the proper temperature is not achieved. These so-called deadlegs are a concern because, if air is present, sterilization probably is not achieved. The only ways to truly detect a deadleg is with temperature measurements or indicator spore strips failing to be killed.
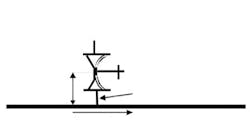
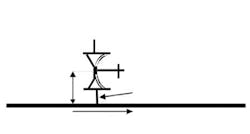
During validation, always place a thermocouple in all suspected deadleg locations to ensure the piping is not a deadleg. My rule of thumb for a suspected free-draining deadleg in a non-vacuum pump system is a length-over-diameter (L/d) ratio greater than 2.5. This ratio is the length of pipe with no steam flow, from the edge of the pipe with steam flow to the end of the pipe that has no flow, divided by the inner diameter of the pipe without steam flow (Figure 1).
Obviously, any deadleg below horizontal is unacceptable, because condensate would not drain. For vacuum pumped systems I have seen L/d ratios of >6 used as a suspected deadleg. Twenty years ago, L/d = 6 was the limit of technology; today, L/d ≤ 2 often is achievable.
2. Don’t use common drains. There is a temptation to tie drain lines together to save piping costs. Do not do this or at least minimize this prior to a steam trap. In parallel lines, sufficient steam may flow through one line, but not the other. It is acceptable to tie drains together after steam traps, if the line is large enough to carry the condensate and some of the steam that bleeds through without creating measurable pressure in that line.
I’ve seen experienced engineers troubleshoot sterilization problems for days until they eventually found that the root cause of the failure to sterilize was two drains tied together prior to a common steam trap.
3. Don’t create stagnant high points. Such points are just potential deadlegs. Steam usually is introduced into vessels through nozzles that are often distant from other nozzles at the top of the vessel. Air seems to be stubborn at high points and is not always predictable. I’ve seen high points that have a 3 L/d ratio not get up to temperature while ones with a 9 L/d ratio did. Due to this unpredictability, it is best to design for positive sweeping steam flow at all these points.
4. Don’t believe that air will flow down based on gravity. Air’s molecular weight is 29 and steam’s is 18. Hence, some people believe that air will naturally fall from high points and be replaced by steam. Don’t believe it! My experience is that it takes steam turbulence to dislodge air and steam flow to carry air out of the system in the sterilization timeframe.
I once visited a company that was designing a new cell-culture reactor. Examining the piping design, I immediately told the designer that the unit would not sterilize properly due to air pockets. The individual explained that the areas I had pointed out would get up to temperature due to air being heavier than steam. I requested he place thermocouples at those locations when he started up the equipment. A few months later the designer informed me that the equipment did not get up to temperature and he had to redesign the piping.
5. Don’t create superheat conditions. Steam sterilization with superheated steam will require dry-heat temperatures and time conditions (much higher temperatures and longer times) and will not follow moist-heat kill kinetics.
Two ways to create dry-heat conditions are:
• Using an external source that heats the saturated steam to superheat conditions. An example that we constantly guard against is the autoclave with a steam jacket. Controls must be put in place to ensure the steam jacket temperature is always lower than the steam sterilization temperature within the autoclave chamber to avoid superheat conditions inside the chamber.
• Subjecting steam to a quick pressure drop (such as via a pressure reducing valve). Even though the steam is at a lower pressure, it still can maintain its heat, creating a superheat condition. Usually, normal heat loss from the system brings the steam back to saturation prior to reaching the equipment being sterilized. I have never encountered dry-heat conditions in equipment being sterilized, but this is what I advised an associate who had: Remove insulation from piping near the reducing valve (if it not likely that someone can be burned); move the reducing valve further up the line; and reduce the steam pressure in stages or put a cooling heat exchanger in place to knock out the superheat.
Not a sterile experience
Designing steam sterilization systems has been a joy over the years. At times, though, it seemed that the processes were like children, always presenting new challenges and reinforcing that I be well disciplined in the basic principles.
The numerous special situations and unique problems in steam sterilization that I have encountered over the years cannot be shared in this short article. However, I hope you’ll find these do’s and don’ts helpful.
William D. Wise is an engineering consultant for Eli Lilly and Company, Indianapolis, Ind. E-mail him at [email protected].