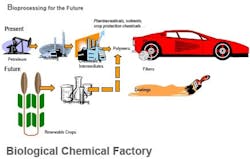
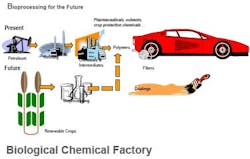
The ability to produce valuable end products via batch biological processes, using such diverse microorganisms as E. coli and other bacteria, algae, mammalian cells, transgenic plants and other host cells, is proving to be a versatile and promising synthesis route for a growing slate of end products (Figure 1). Because such processes rely on the fermentation of renewable carbohydrate feedstocks (from cane, beets, corn, grain and other sources), they have the potential to offer an environmentally friendly and less costly alternative to conventional synthesis routes that are based on petroleum-based feedstocks, which face supply and price pressures (Figure 2).
Figure 1. Biprocessing provides an increasing variety of products, including some commodities, with many more likely.
Figure 2. Crops and agricultural wastes offer a source of renewable feedstocks and more control of raw material costs.
While fermentation-based syntheses were once reserved for producing high-value specialty chemicals and biopharmaceuticals (whose end products may command prices on the order of $400,000 for a 5-mL vial), bioprocess routes now are gaining increasing attention for commodity products. For instance, commercial-scale bioprocess facilities are already producing: vaccines and therapeutic pharmaceuticals (such as Amgen's Epogen and Wyeth's Mylotarg), food products (L-phenylalanine, a building block for NutraSweet), and food-grade additives (such as the algae-derived fatty acid DHA & ARA from Martek Biosciences, which is used as a nutritional additive). Other specialty and commodity biochemical facilities are in the works. For example, BP and DuPont are teaming up to commercialize bio-based butanol as a gasoline blendstock in 2007 (CP, August, p. 15).
Scale-up challenges
While the scale-up of any chemical process can involve a host of issues, the challenges are compounded when the process involves batch fermentation. Due to the typical fragility of the engineered microorganisms, large-scale fermentation vessels must be designed with the ability to:
- remove the heat buildup that results from metabolic processes;
- manage agitation and mixing with minimal shear damage;
- effectively control the highly variable liquid flowrates and turndowns that are associated with batch fermentation; and
- execute safeguards and sterilization techniques to guard against potential contamination.
The engineering challenges are more acute when the fermentation process will be used to make commodity-type chemicals. Because such products don't command premium prices like higher-end specialty chemicals, food additives and biopharmaceuticals, their production facilities often are forced to make engineering tradeoffs in the face of capital, maintenance and operating cost constraints and leaner profit margins.
Additional challenges arise because emerging (non-mature or unproven) synthesis routes often exhibit a high degree of change throughout the scale-up and design stages. With relatively limited project experience with a given route to draw upon, the design team must anticipate and manage changes to the design and construction specifications to minimize cost creep and keep the project on schedule.
One of the most commonly made mistakes during the design of bio-based manufacturing processes is the failure to adequately integrate the experience, expertise and proven techniques developed by the pilot-plant engineers, facility microbiologists and chemists into the criteria for the overall flowsheet, equipment specifications, process and instrumentation diagrams, and waste-handling systems. The members of the design, operations and maintenance teams that will set these criteria are generally added closer to startup and face tremendous task and time constraints to be ready for commercial operation, diminishing their availability for technology-transfer efforts. However, during the specification of commercial-scale equipment and controls, it is crucial to study and adapt the administrative and manual tasks carried out during pilot-scale operations, such as those related to closed-vessel policies, material handling, cleaning, waste handling and other operational aspects. A well-integrated team approach, with a common project view of the need to balance cost constraints against sterility needs, is essential.
"Bio" issues
When producing pharmaceuticals and food additives, product contact streams are regulated by the U.S. Food and Drug Administration and, possibly, by the U.S. Department of Agriculture in the case of biosynthesis. By comparison, chemical facilities are only regulated to keep the genetically engineered organism out of the surrounding environment.
Experience shows that the performance characteristics of various organisms — even those already in use to produce a given product — can be improved through ongoing bioengineering efforts. As a result, "next generation" bugs (such as those with enhanced metabolic activity or less sensitivity to process conditions) are constantly being pursued, to increase the yield of the target product, decrease the batch cycle time, reduce the amount of effluents or undesired byproducts, and cut energy consumption.
While such improvements are worthwhile, they may necessitate changes in equipment or utilities. For example, increased metabolic rates can enhance throughput, provided the higher heat generation can be controlled within the required temperature band, and agitation and delivery systems suffice to deliver needed nutrients and oxygen to the more quickly multiplying organisms.
A common framework
Irrespective of the microorganisms used or the end products produced, most fermentation-based facilities employ the same basic production blocks. And all commercial-scale bioprocess facilities can be roughly divided into two sections:
- The upstream biosynthesis operation, where the desired end product is made, typically involves highly proprietary methods and calls for rigorous sterility requirements.
- The downstream portion employs a site-specific mix of widely used chemical-engineering unit operations to extract and purify the target product, and appropriately dispose of all waste streams.
The particular engineering requirements (and challenges) associated with each of these two distinct portions differ, but must be tightly integrated during process design to ensure the most-cost-effective plant operation.
Fermentation. Each of multiple fermentation vessels required by a commercial-scale facility will have its own particular design and operating requirements. These include: the need to introduce the fermentation broth, sterile air (both to maintain the required dissolved oxygen levels and provide air lift for low-shearing mixing inside the vessel) and sterilized nutrients (such as vitamins, amino and fatty acids, minerals and even antibiotics that ensure the health and maximize the productivity of the microorganisms). When air lift in the vessel can't provide sufficient mixing, the fermenter may be equipped with low-shear agitation devices.
Fermentation vessels must also be designed to ensure adequate heat-removal capabilities (to handle heat produced by the metabolic processes) and promote cooling as needed (to maintain the narrow temperature range that can be tolerated by the bioengineered organisms). Sufficient safeguards must also be in place (both through design elements and operating procedures) to guard against contamination and cell mutation. These include double-block and bleed valves, and steam-in-place (SIP)/clean-in-place (CIP) systems. Meanwhile, the variable flow rates associated with different stages of the organism's metabolism and growth cycle, and the required cleaning cycles have tremendous design implications for the turndown necessary for process parameters (including flow and pressure). All of these factors complicate the internal geometry (in terms of baffles and agitators, for instance) of the vessel, as well as the number, location and type of tank nozzles and ports needed.
In addition, commercial-scale fermentation vessels must be equipped with a variety of advanced instruments, sensors and transmitters to monitor everything from pressure, level and temperature inside the fermenter to pH, dissolved oxygen and nutrient levels within the fermentation broth. In some cases, the number of in-line monitoring devices needed can be reduced by using external lab sampling or indirect relationships between key operating parameters. The appropriate number and location of the analytical instruments and in-process checks must also be reconciled against capital and operating cost constraints, and sterilization concerns (an increasing number of instrument ports raises the contamination risk to the bioreactor).
Because fermentation vessels and other specialized equipment components often require long lead times, the ability to make a firm commitment to the desired design specifications in advance — and withstand the urge to make subsequent changes later — will help to control costs and minimize delays.
Production and recovery of derived product. During fermentation, the desired product ends up in the fermentation broth (excreted from the microorganisms or within the cell body). At the end of each batch fermentation, the microorganisms are destroyed; the product is separated and purified; and the dead cell bodies, unreacted carbohydrate feedstock/nutrients and byproducts are removed, concentrated and neutralized prior to disposal.
Purification and concentration. A combination of classical separation operations (such as filtration, evaporation, ion exchange, distillation, crystallization, liquid/liquid extraction, spray drying and direct reaction) are typically used to purify and concentrate the product from the fermentation broth. Increasingly, newer separation techniques (such as liquid chromatography, liquid and catalytic membranes, and supercritical fluid extraction) are finding a role in commercial-scale bioprocess operations.
Waste handling. The dead cell bodies and other solid waste, and the high biochemical-oxygen-demand (BOD) aqueous streams produced throughout the process must be disposed of properly. They must be concentrated and neutralized prior to disposal; an aqueous waste-pretreatment facility is almost always required to reduce the bioload prior to discharge to a landfill or publicly owned treatment works (POTW). The specific handling and disposal requirements are ultimately dictated by the biosafety classification of the microorganisms in the waste stream.
Residual high-BOD aqueous waste streams result and are typically treated in onsite aerobic or anaerobic digesters prior to being sent offsite to a POTW. Aerobic digestion is economically applied for BOD up to 10,000 ppm. Anaerobic digestion is generally used from 8,000 ppm and up, and almost always when BOD exceeds 15,000 ppm. (Anaerobic systems will require further processing when discharging directly to a river but are commonly used for city sewer discharge.)
Pitfalls and opportunities
Several issues deserve particularly close attention during the design and construction of bioprocess facilities, to streamline the overall process, minimize rework and contain costs.
Sterility considerations and tradeoffs. The microorganisms that are used during biological-treatment processes at water- and sewage-treatment plants are notoriously hardy and can handle widely varying operating conditions. By comparison, microorganisms that have been genetically modified to yield a desired pharmaceutical, food or chemical product are typically viable over only a very narrow range of operating conditions and cannot withstand large or sudden variations in temperature, pH, dissolved oxygen, nutrient level, agitation rate and other critical operating parameters. The extreme sensitivity of these highly evolved yet fragile organisms — which has earned some the nickname "metabolic cripples" — creates unique design and operating challenges, particularly when it comes to maintaining close control over all of the critical operating parameters within vessels whose capacities generally exceed 50,000 gallons.
As noted earlier, the microorganisms in a given fermentation broth are highly susceptible to the presence of impurities. Therefore, it is critical to guard against potential contamination. The most common sources of contamination are invasion by phage infections (rogue cells unique to each facility) and mutations within the bioengineered organism population. Such occurrences not only lead to the disposal of the valuable fermentation batch but also call for an immediate shutdown for sterilization and cleanout.
Master cell cultures are typically created and maintained offsite to minimize the possibility of mutations and phage infections. Nonetheless, the facility's approach for sterilization, cross-contamination prevention and routine cleaning must be identified early to ensure the piping and equipment configuration meets the intended cycle time and sterilization objectives.
When it comes to addressing sterility concerns, bioprocess facilities that produce high-value specialty chemicals and pharmaceuticals often can easily justify the very best sterile equipment and components. However, commodity biochemical facilities will not be able to afford such luxuries as large-pipe-diameter diaphragm and sanitary valves, sanitary tubing, specialized aseptic fittings, removable components and instruments, automated SIP/CIP systems, and ultra pure water for process use. Indeed, bioprocess facilities producing lower-margin commodity chemicals are often forced to balance budget-related engineering tradeoffs without compromising process sterility. This can be accomplished by using appropriate lower-cost equipment components (e.g., standard industrial valves with welded pipe connections) with more rigorous SIP/CIP procedures and valve preventative maintenance.
The compressed air system is one important example of an element demanding close attention. It provides the huge volumes of air used to deliver oxygen and air-lift capabilities to the fermentation vessel. Designing systems that adequately filter airborne contaminants and bacteria, and remain dried (to avoid entrained condensate carrying bacteria through filters) requires design rigor. This is particularly crucial for facilities operating in hot, humid climates. Retrofitting after the fact is very costly.
Extreme operating variances. Due to the cyclic nature of batch fermentation, bioprocess facilities typically have enormous tankage requirements and highly transient flow rates, which call for very complex pump and valve assemblies (both in terms of the number of components needed and their necessary turndown capabilities). To cost-effectively achieve turndown rates as high as 15:1 (flow requirements that can vary from 1,500 gpm to 100 gpm from a single device), bioprocess facilities often contain a significant number of pumps equipped with variable-frequency drives (VFDs) and extensive control-valve systems, both of which increase capital costs and software-programming effort.
Operating costs. Recycle and energy reuse are the standard for successful biologic commodity products. Commercial-scale fermentation facilities handle enormous volumes of water and steam (with varying composition and temperature) from fermentation, purification, evaporation and cleaning systems. Dynamic/transient mathematical modeling programs offer an invaluable tool to help designers identify the water and energy pinches and, thus, to strategically combine water streams of varying composition and temperature to achieve maximum water and energy recovery. Such modeling can ensure that the overall flowsheet has the appropriate number and size of tanks and piping arrays to maximize water and energy reuse. With non-mature processes, significant changes in these areas can be expected as new information surfaces throughout design.
The location of the facility provides another opportunity for cost minimization. For instance, the ability to co-locate a fermentation-based manufacturing plant near a low-cost source of carbohydrates, such as a facility that is processing grain, corn, beets, sugar cane and other farm-derived products, can help to reduce both raw-material and transportation costs.
Bioprocesses require removal of large volumes of water. So, considerable operating savings can be realized by opting for today's highly efficient separations technologies, such as evaporation with mechanical vapor recompression and multiple-effect evaporators. In general, low-energy evaporation is essential in commodity bioprocesses to keep operating costs aligned with product costs. However, getting from vendors design information essential in sizing utilities and building takes time; expect three to four months to pass before usable information is received. This, along with appropriate expediting resources, should be factored into the original design schedule and staffing.
Proper design of the waste-disposal facilities can also help to contain operating costs. Typically, the initial solids separation (cell/product) is done in complex unit operations, and the solids are further dried using standard press, plate, belt or drum dryers before being sent to a landfill for disposal.
Biosafety. The U.S. Environmental Protection Agency and the National Institutes of Health have issued guidelines for handling of many of the commercial microorganism strains. In addition, in the U.S., Toxic Substances Control Act regulations establish procedures for commercializing new or modified strains.
The biosafety classification of the microorganism used in the fermentation process will determine what level of containment is required for operations such as sampling, offgas venting and waste disposal to minimize the potential for biohazard risk to personnel and the environment. Certain criteria must be met for the containment and deactivation of the microorganism — using "any combination of engineering, mechanical, procedural, or biological controls designed and operated to restrict environmental release of viable microorganisms from the structure."
Long-lead equipment. While the design and operation of liter-scale fermentation vessels used during bench- and pilot-scale testing is relatively easy, considerable complexity enters the picture when the process makes the jump to commercial-scale capacities. Such facilities typically require a mix of ASME- and API-specified vessels. Fermentation vessel design must account for the high pressures and temperatures needed for sterilization as well as the large capacities required, up to several hundred thousand gallons. Such large-scale vessels require field fabrication, with internal finishes often calling for extensive hand polishing and passivation. Material lead times and site construction presence must be factored in during the planning stages. Other long-lead equipment typically include large vendor packages needed for purification — such as filtration skids, distillation and extraction columns, mechanical recompression equipment and spray dryers. Insufficient planning related to transportation, vessel fabrication and erection, welding and finishing — particularly when the work onsite could interfere with the timely execution of foundation preparation and other construction activities at the site — is often to blame for delayed startup.
Successful scale-up
The identification and genetic engineering of a suitable organism, followed by prudent piloting certainly is crucial to success with bio-based manufacturing. So, too, is effective technology transfer from the development effort and adequate attention to practical design issues. As is so often the case, a scale-up strategy that combines integrated teamwork with solid engineering efforts can go a long way to minimize costly rework and delays, and help today's promising manufacturing routes based on renewable feedstocks to achieve their full commercial-scale potential on time and on budget.
John L. Shaw, P.E., is a senior technology manager for CH2MHill Lockwood Greene, Spartanburg, S.C. E-mail him at [email protected].
Scott A. Rogers, P.E., is a process engineer for CH2MHill Lockwood Greene, Spartanburg, S.C. E-mail him at [email protected].