Disposable Equipment: Single-Minded Quest Continues
More Content on Disposables you May Like. . .
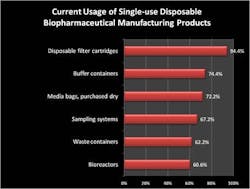
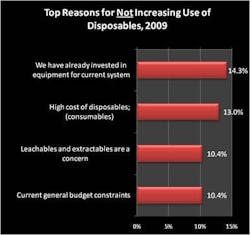
Single-use equipment continues to make strong inroads among bioprocessors, according to a new study — the "6th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production," published in April by market research firm BioPlan Associates Inc., Rockville, Md. Acceptance and prevalence of disposables in the industry is rapidly increasing as companies gain experience with the devices, notes Eric Langer, president of BioPlan. The 210-page study provides data on 10 critical areas associated with biopharmaceutical production, gathered from 443 production executives at drug developers and contract manufacturing organizations in 35 countries. "In our study, we evaluated biomanufacturers' current usage of single-use/disposable systems. Disposable filter cartridges were the most commonly listed disposable items, noted by 94% of our respondents (equal to last year's usage data). On the whole, however, the survey responses suggest that almost every kind of disposable has penetrated the market broadly (Figure 1). Fully 61% of our respondents reported using at least one disposable bioreactor, and 74% reported using buffer containers, and 56% were using a disposable mixing system. Even for filled media bags, acceptance in at least some part of the production network was reported by 59% of those we surveyed (from 48% in 2005)." "This year, decision makers' objections to disposables declined both in 'quantity' and importance. For example, we asked respondents about their most important reasons for not increasing their use of disposables (Figure 2). Factors such as how regulatory agencies would treat leachables and extractables issues have consistently been near the top of the list. However, in this year's study, the number of respondents citing regulatory worries as a primary concern for restricting their adoption of disposables fell to 10.4%, down from 16.6% last year. In addition, in previous years lack of disposable equipment that meets process requirements, and breakage of bags and loss of material were major concerns. This year, these issues were no longer primary concerns to respondents," notes Langer.