Hot-water sanitation and continuous high-temperature processing are essential to product quality and membrane system performance in numerous applications, including production of United States Pharmacopeia (USP) water and water for injection, enzyme manufacturing and chemical production processes that require low bacterial levels or limits. Many industries such as pharmaceutical and biotech must rely on, or are turning to, chemical-free hot-water sanitation to meet product quality requirements and save time and resources.
Hot-water sanitation effectively protects against detrimental bacterial growth on the membrane surface, thereby limiting membrane replacement costs and the associated system downtime. Furthermore, continuous high-temperature operation during the processing of valuable materials improves product yields while remaining economical and energy-efficient. Additional benefits of high-temperature operation include:
Cleaning and sanitation. Membrane systems treating biologically active feeds can be sanitized by periodic exposure to temperatures as high as 90 Degrees C, without the use of chemicals.
Continuous processing. High-temperature processing of industrial liquids and pure water results in reduced energy usage and operating expenses.
Efficiency and cost-effectiveness. High-temperature operation eliminates capital and operating expenses associated with feed stream cool-down prior to membrane processing.
Membrane technologies
Various high-temperature membrane applications currently are available to purify hot boiler water and evaporate condensate, dewater hot product steams, dewater hot waste streams and more. Today's membrane technologies include high-quality membrane materials that maintain pore size and separation quality when exposed to high-temperature fluids (from 50 Degrees C to 90 Degrees C) and adhesives that are capable of holding a membrane together over extended and repeated exposure to hot fluids.
Reverse osmosis (RO) technology has been used for decades to generate makeup water for processes and pharmaceutical-grade water. Advances in RO membrane technology and system design have included polyamide thin-film composite membranes, two-pass vs. one-pass RO systems, multistage vs. single-stage pumps, sanitary piping and instrumentation and, more recently, hot-water sanitation membranes.
To the disappointment of many, however, RO systems do not "sanitize" feed water streams, nor are they capable of completely and continuously rejecting bacteria from feed water sources. Common bacteria species are physically larger than the average pore size of an RO membrane and are rejected from the feed stream into the concentrate stream. However, inconsistencies in the membrane pore size make absolute rejection impossible.
Bacteria in membrane systems
A relatively large membrane surface area, ambient operating temperatures and the absence of chlorine or another disinfectant combine to create a microbe breeding environment on the permeate side of RO membranes. Bacteria counts taken from water streams typically are representative of the "loose" microbes in the stream and not necessarily of microbes that can exist in a biofilm within the water system.
Biofilms form naturally, but can be mitigated with appropriate fluid velocity, smooth sanitary pipe designs and periodic and appropriate sanitation processes. However, biofilms have proven to be quite resistant to many chemical sanitation processes because of their protective boundary layer. Studies demonstrate biofilms can survive even after 60 minutes of exposure to chlorine-based sanitizing chemicals. Therefore, the prevention of biofilm formation is a primary goal with respect to "regular and periodic" sanitation processes.
To exhibit better system control over microbe levels, end-users frequently employ routine chemical and hot-water membrane sanitation processes often based on historical bacterial growth rate data.
Chemicals used in RO membrane cleaning procedures include low- and high-pH cleaners for the removal of hardness scale, or calcium carbonate precipitation, and organic deposits. Some microbiological control also is achieved as a secondary benefit. Some common chemical polyamide membrane sanitizing agents include 0.5 percent formaldehyde, peracetic acid and hydrogen peroxide.
The USP and the recently published International Society for Pharmaceutical Engineering (ISPE) Baseline Guide on Water and Steam Systems regulations allow end-users latitude in determining sanitation processes. Recently, many chemical facilities have put into place hot-water processes, as a result of the industry's concern over "added substances" in the water stream.
Although many chemical sanitation processes might be somewhat ineffective against biofilms based on specific chemicals, exposure time and frequency of application, the sub-boundary layer biofilm cannot escape exposure to sanitizing temperatures during a hot-water sanitation process.
Hot-water sanitation process
It is important to understand that the term hot-water RO describes a system designed for periodic sanitation with hot water, rather than an RO system operating at elevated temperatures. Effective sanitation with hot water is accomplished through an appropriate combination of exposure time and temperature. Although an optimum sanitation target point has not been established offically, the industry has "standardized" on 80 Degrees C for 30 minutes to 60 minutes.
A primary use for hot-water RO sanitation is to inactivate viable microbes. Endotoxin reduction is not achieved as a direct result of the hot-water sanitation process. Based on the feed-water source, system operating conditions and the end-user's preventive maintenance practices, traditional chemical cleaning processes might still be required. Generally, hot-water cleanings are performed more frequently than chemical cleanings, based on microbe counts and/or a regular sanitation schedule.
Sanitation of an RO system using hot water commonly involves incorporating a heat exchanger(s) into the traditional clean-in-place (CIP) system to gradually, at a controlled rate, heat and cool water circulating through the membrane system. Membrane manufacturers commonly stipulate a controlled heating and cooling rate (5 Degrees C per minute) to protect against irreversible damage to the membrane and ensure the system's long-term performance.
It is recommended that plants take this six-step approach to hot-water sanitation:
1. Perform ambient-temperature, low-pH chemical cleaning for scale removal or for other foulant as necessary.
2. Heat to 80 Degrees C at a rate not to exceed 5 Degrees C per minute (approximately half an hour).
3. Maintain feed pressure to RO system below 40 pounds per square inch gauge (psig).
4. Maintain cross flow so the pressure drop is less than 2 pounds per square inch drop (psid) per membrane.
5. Circulate water through the equipment at 80 Degrees C (half an hour).
6. Cool to 25 Degrees C at a rate not to exceed 5 Degrees C per minute (approximately half an hour).
Component design and system requirements
When designing a hot-water sanitation RO system, the designer must consider every water contact component to ensure temperature compatibility. Major components include membranes, membrane housings, pumps, pipe/fittings/ gaskets, valves, instruments and cleaning (CIP) tank.
Of these components, membranes are the most complex and costly. RO membranes used in hot-water systems are similar to ambient-temperature membranes in that they are cast in a polyamide material and constructed in a thin-film composite configuration. However, to withstand the elevated operating temperatures, they are manufactured with special adhesives, permeate tubes and connectors.
Hot-water sanitation membranes
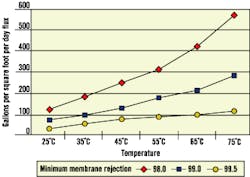
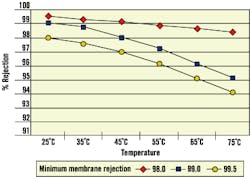
Like ambient-temperature membranes, hot-water membranes exhibit different operating characteristics at higher temperatures. For example, flux rates increase while rejection rates decrease (See Fig. 1 and Fig. 2). Therefore, when considering continuous operation at elevated temperatures, plants also should consider dissolved solids loading to post-RO treatment ion exchange equipment.
Figure 1. Membrane Flux vs. Temperature
Figure 2. Membrane Rejection vs. Temperature
The original operating parameters of the membrane are essentially recoverable after the initial hot-water sanitation at 55 Degrees C or higher. However, after the first sanitation, membrane flux at ambient temperature is permanently reduced by 50 percent. Subsequent hot-water sanitations have no further appreciable effect on ambient temperature flux rates. To ensure minimum flow requirements are satisfied in the long term, hot-water systems are designed with significantly more membrane area than ambient-temperature systems with equivalent design flow rates.
System design options
Several hot-water sanitation membrane system designs currently are available. Among the key and minimal design criteria for a dependable system are:
Appropriate membranes.
Appropriate materials of construction.
Automated programmable logic controller/proportional integral derivative (PLC/PID) loop controlled heating and cooling processes.
Automated valving to minimize operator interface.
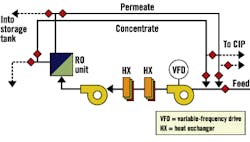
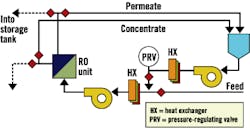
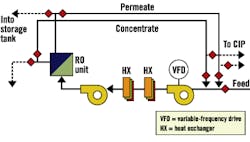
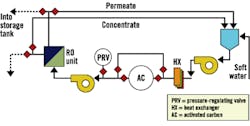
Fig. 3, Fig. 4 and Fig. 5 provide schematics for three basic system designs. In each design, a method to closely regulate feed pressure to the membranes during hot-water sanitation is employed by either a pressure-regulating valve or a variable-frequency drive for direct pump control. Fig. 4 and Fig. 5 provide designs with independent heating and cooling heat exchangers. Fig. 5 uses a common and dual-rated heat exchanger for both heating and cooling processes. In each case, the "hot" heat exchanger also serves to preheat RO feed water during normal operation.
It is assumed Fig. 3 and Fig. 4 use either sulfite chemical injection or ultraviolet (UV) technology for dechlorination. When using activated carbon (AC), as shown in Fig. 5, both the RO and AC could be hot-water sanitized during the same process.
Figure 3. Two-Heat-Exchanger System
with CIP/sanitation Tank, On-line/Off-line HXs
Figure 4. Two-Heat-Exchanger System with No Tank
Figure 5. One-Heat-Exchanger System
with Soft-water Tank, Feed-Water Cooling
Conclusion
Today, membrane technology has advanced to the point where it can ensure reliable, predictable performance for use in high-temperature processing applications. In addition, the systems themselves can be automated to perform dependably and safely. CP
Wise is the application engineering manager for engineered equipment at Osmonics Inc., Minnetonka, Minn. He can be reached at (952) 988-2277, or via e-mail at [email protected].