Environmental Protection: Scientists Supposedly Solve Silver Snafu
A brief Internet search for “using silver in fabrics” reveals a host of companies manufacturing everything from sportswear to bedding, gloves, medical scrubs and towels.
The common factor among them is the use of compounds containing silver (Ag+) ions against the predations of microbes, notably those that cause body odor and irritating skin conditions.
However, silver has its problems. For example, it can be difficult to apply to soft and porous substrates, such as natural and synthetic textiles, without negatively impacting the textile. Many antimicrobial coatings require complex application strategies and have undesirable qualitative properties that can limit their use. These include high absorbance of visible light and corresponding discoloration, clogging of textile pores, or the incorporation of potentially toxic compounds.
Perhaps the most concerning problem is heavy leaching during use or washing. Silver, a biocide, poses a threat to life in river and sea sediments if it enters the water cycle.
A 2018 report by The Swedish Water & Wastewater Association (Svenskt Vatten), Bromma, Sweden, highlighted the problem. The organization tested silver-containing garments described as anti-odor. Every single one had experienced leaching, ranging from 31–90% after ten washes — the latter from a pair of sports leggings. The median value was 72%.
This reinforced a previous report by the Swedish Chemicals Agency that found two out of the three silver-containing products it tested had no antibacterial effect after ten washes. In fact, the agency concluded it was almost down to luck if any garment still performed after several washes.
The Svenskt Vatten report also pointed out that research showed silver seemed to reduce the level of sensitivity/resistance of microbes to antibiotics.
Now, researchers at the University of Tokyo believe they have made a breakthrough in tackling silver leaching.
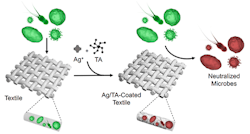
Writing in a recent issue of Nature Scientific Reports, researchers claim to have found the first cost-effective and convenient way to apply a silver-based antimicrobial clear coating to new and existing textiles.
The coating uses the polyphenol tannic acid (TA) found in chocolate, wine and other familiar super-stainers to bind silver in such a way that a garment can be washed multiple times without losing its antimicrobial and anti-odor properties.
This Ag+/TA coating also is completely clear, so it doesn’t discolor textiles, and is only 10-nm thick.
Two ways of applying the coating were tested. In the first, of possible interest to commercial fabric manufacturers, textiles are bathed in the Ag+/TA mixture. The second, which the authors note could suit small-scale settings, involves spraying the garment first with silver nitrate and then with the polyphenol TA binder. An obvious advantage here, they point out, is people can add the coating to existing items of clothing. Both processes take about 20 minutes.
Because of the broad-spectrum antimicrobial protection of the Ag+/TA coatings observed against viruses, bacteria, and fungi, the authors foresee their “facile, rapid and relatively sustainable” approach will find use in various medical, health and lifestyle applications.
Figure 1. Ag+/TA coating could be done by anyone, not just in tightly controlled industrial settings. Source: Ejima et al, 2002.
Interestingly, the article’s ethics declaration notes one of the authors is an inventor on a patent application with aspects related to this work and is pursuing commercialization of the technology.
While the Tokyo team believe they have solved the leaching issue, others are keen to do away with silver completely.
Parx Materials, Rotterdam, The Netherlands, for example, has launched a pair of anti-odor socks that use zinc as the key ingredient.
The Dutch company focused on zinc because of its well-documented involvement in the human immune system and its ability to fight off bacteria and viruses.
The socks are fabricated with a mix of polypropylene and polyethylene terephthalate yarns, each impregnated with slightly differing versions of the company’s patented zinc-containing Saniconcentrate polymer. The metal is integrated in its ionic form, but as an intrinsic part of the polymer it is fixed inside the material with no releasing mechanism or migration.
In tests with three different microorganisms and a selection of different incubation times, it was found that antimicrobial characteristics remain solely on the surface of the material and no zinc leaches out.
To gather some user experience, 500 pairs of socks were tested by interested members of the public. According to the company, some reported the socks contained no foul odor even after using them for 20–30 consecutive days.
Seán Ottewell is Chemical Processing's editor at large. You can email him at [email protected].