Certain processes — such as those for electronic chemicals, monomers feeding high-yield polymer streams, and high-purity steam generators — routinely require analysis of ppb concentrations. Getting accurate laboratory results demands special configurations and handling. Factors that may affect sample validity include absorption of chemicals onto tubing or container surfaces and trace contaminants.
Sampling methods for these systems require special attention to sample integrity. While specifics vary depending upon the process, many common factors come into play. The most important of these often revolve around getting a representative sample.
At ppb levels, surface interactions between the process and the piping can become important. In some systems, the piping surface won’t reach equilibrium with the process until weeks or even months of exposure. Dynamic changes also influence compositions. Specific components may absorb onto the surface of the pipe; then, as bulk concentrations drop or conditions change, they desorb.
Surface sorption effects depend upon the ratio of surface area to flow cross-section: the larger the diameter of the sample system piping, the lower the surface-area/cross-section ratio — and the impact of sorption on sample integrity.
However, conventional sampling systems often include a connection segment that normally has no flow. The need to flush this segment prior to sampling incurs a cost in lost material that can pose a significant economic penalty for systems with high-value compounds. Opting for a smaller-diameter sampling line minimizes the cost.
Two key steps help improve sample integrity. First, select sampling system materials that will prevent or reduce sorption effects. Second, use a “fast loop” or sidestream sampling system with continuous flow at all times.
Materials’ selection depends upon the system. For example, many electronic chemicals systems work well with stainless steel pipe. As long as the correct grade is selected and properly conditioned before service, sorption effects are minimal. In contrast, systems sampling for hydrogen sulfide at low levels never should use stainless steel because the sorption effects can be very significant. Good choices for low-level hydrogen sulfide monitoring are polytetrafluoroethylene-lined pipe for moderate conditions and high-nickel alloys for more severe services. Investigate the service to understand your choices.
A fast loop provides a parallel path for flow; the continuous flow prevents dead legs. The loop usually contains the sample container. When a sample is needed, valves isolate the container, and then a new one immediately is placed into service. Extremely high-purity systems may include purging of the new container to prevent material from it contaminating the system.
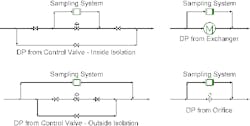
Figure 1. Such loops always must be installed in parallel to a pressure drop.
The fast loop must be placed in parallel with a pressure drop (∆P). Figure 1 shows some commonly used options.Having the fast loop in parallel with process equipment such as a control valve or heat exchanger usually minimizes operating and installation costs. However, it also creates a new flow path. This may change potential consequences of failures. It also may make more complex the procedures for equipment isolation while the unit is running. So, sometimes, placing the loop in parallel to operating equipment may not be a good choice.A better option in such cases may be to install a restriction orifice specifically to generate flow for the fast loop. Such a stand-alone package provides more flexibility in installation but the orifice will add pressure drop in the system that may increase energy costs or reduce unit capacity. If necessary, the orifice can be installed between flanges or welded-in if flanges are considered leak or contamination points.The configurations shown all assume simple single-phase sampling. If the system is two phase, getting a representative sample becomes much more complex. Isokinetic sampling (see: “Don’t Be Fazed by Multiphase Sampling") is required to get the slipstream. Piping configurations must prevent phase separation from creating unrepresentative samples. The position and orientation of sampling nozzles also can significantly affect analyzer results (see: “Properly Position Sampling Nozzles").Without good samples, controlling the process can be difficult and expensive mistakes can occur. While specifics of high-fidelity sampling systems vary with the process, accounting for sorption effects and using fast-loop systems can help ensure getting truly representative samples.ANDREW SLOLEY is a Contributing Editor to Chemical Processing. You can e-mail him at
[email protected]