Dow engineer wins the 3rd annual Plant Innovation Award
Engineers rarely get the peer recognition they deserve. So, once again this year, Chemical Processing is proud to honor an engineer who met a challenge with a creative solution.
This year’s award goes to Bridget Schoonover, senior improvement specialist for Dow in Freeport, Texas, who was nominated by Steve Gluck, a scientist, and Ralph Pons, an improvement leader and project champion, at Dow’s Freeport, Texas, operations. She led a team that validated and implemented a solution to a source of lost revenue at the plant: a waste stream. Her team included (from left to right below) Johanna Graishe, Sowmya Akula, Phil Listak, Jamshid Mohebalian, Arturo Lopez, Joel Welch, Wes Heinlein, Robert Prescott, Kristin Cole, Ralph Pons, Steve Gluck, Steve Smith, and Glenn Lord.
Absent from the picture are: Stephen Jones, Greg Bond, and Mike Rixon. As a result of Schoonover and her team’s efforts, this waste is recycled thereby reducing the potential for pollution, cutting treatment costs and providing supplemental feedstocks.
The process
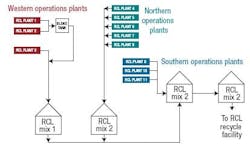
Dow creates chlorinated organic byproducts (RCls) in several of its manufacturing facilities at Freeport. Dow has an existing facility that recovers the chlorine as an HCl feedstock. In this process, byproduct RCls are collected from several operational areas, blended and transferred via 10 miles of pipelines to the RCl recycle manufacturing plant (Figure 1).
Figure 1. The chlorine reclamation system at Freeport relies on carbon steel.
The RCl recycle strategy is to maximize consumption of all qualified RCl streams as feedstocks. The facility, which recycles the RCls, was designed to consume a predetermined feedslate of these materials.
The problem
RCl Stream 3, a large volume feed planned for consumption in the recycle facility, had been removed from the feedslate in 2004 due to concerns about corrosion to carbon steel in the raw material storage and transport systems. Without Stream 3’s volume, the feedslate couldn’t meet design capacities and was inadequate to maintain full rates during high production.
Dow was evaluating an option to invest $6 million to build a dedicated transport and storage system to get Stream 3 back into the feedslate. The opportunity to resolve corrosion concerns using trace chemistry within the existing feeds and Stream 3 was presented by R&D. Because the storage and transport system served several business units, the risk of damage to the single pipeline would have affected the reliability and profitability of the entire Freeport facility.
“The potential presence of water and HCl in the mixed stream created a significant imbalance between generation and disposition of the RCl streams,” says Brad Fedorchak, environmental operations production leader. “This imbalance not only limited the recycle of material, it impacted profitability, and placed a significant asset-utilization burden on the thermal treatment units and limited production capabilities on site. This was not a sustainable position for the manufacturing units on site to be in and thus the six-sigma project to understand the water generation mechanism and develop a control strategy was initiated.”
Historically most organic waste producers find that storing RCls in a wet environment can be corrosive to carbon steel equipment because these streams often contain trace levels of HCl. Hence, Dow, which had constructed its the RCl mix storage and pipeline systems of carbon steel, set a specification that all RCl feeds contain <50 ppm water. Introduction of Stream 3 to the mixed RCl system in 2004 resulted in the detection of 300 ppm to 700 ppm water in the blended material. Therefore, Stream 3 was removed and a series of root-cause investigations proceeded.
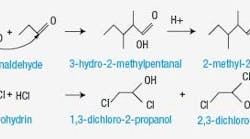
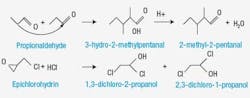
As with many problems involving chemistry, the high concentration of water in the RCl mix wasn’t fully understood. Max Lee, senior research chemist, recalls, “the production people were questioning the analytical method accuracy because dry streams were blended resulting in a wet tank stream.” A series of research studies were conducted to understand the water formation mechanism and to develop a method of mixing Stream 3 with other feeds to the HCl unit. The ultimate goal was to reduce the corrosiveness of the feeds. Research revealed that trace HCl in the other RCls was catalyzing a reaction with certain oxygenated components in Stream 3 to create the water. Further work identified the mechanism for these reactions: aldol condensation of propionaldehyde, which was found as a waste in the Stream 3 (Figure 2).
Figure 2. Discovering the source of the excess water in the product was the first step in eliminating the corrosion problem.
The plan of attack
Jeff Birdwell, process chemist, said “water isn’t the problem, corrosion is. Why not use the excess epichlorohydrin in the stream to consume the HCl?” Subsequent laboratory mixing and corrosion studies revealed that epichlorohydrin, also contained in Stream 3, did consume the HCl and yielded a noncorrosive mixture.
At this point, a six-sigma project was chartered to evaluate the feasibility of scaling this knowledge up to manufacturing scale. Schoonover led this project and assembled a project team consisting of representatives from six manufacturing plants, subject matter experts, as well as analytical and supply chain personnel.
Click to enlarge chart.
The team repeated the initial lab studies on a mid-size (5,000-gallon) facility, conducted an extensive set of statistically designed laboratory experiments and a comprehensive Failure Modes and Effect Analysis (FMEA). Schoonover used this information to conduct a business economic analysis comparing 14 different improvement alternatives to mitigate different risk scenarios. Presentation to manufacturing leadership secured support to pursue full scale implementation. The $6 million dedicated storage and transport system alternative was abandoned in favor of spending $500,000 to improve the mixing equipment and install units to deliver pure epichlorohydrin if the system ever ran short of Stream 3 supply.
The control plans and a revised supply-chain-management model were created using the laboratory results. By conducting an exhaustive study of all possible blends, the team had been able to show that the corrosion rate could be consistently minimized regardless of water concentration when epichlorohydrin is contained in the mixture and the HCl content is lowered by more than 90%. Because epichlorohydrin exists in concentrations of 6% to 12% (by weight) in Stream 3, an epichlorohydrin mass-balance calculation was incorporated into the RCl supply chain inventory management methodology. To predict and control the new mass balances properly, additional analytical capabilities were implemented in the source plants and mixing operations to monitor epichlorohydrin, HCl and water.
“We needed a solution which addressed a complex operation with a easy-to-use solution, addressed risk concerns associated with historical biases and ensured sustainability with zero allowance for failure,” says Schoonover. “Six sigma methodology, a constancy of expectations from sponsors, and pursuit of sufficient data to alleviate stakeholder fear were the elements that allowed us to complete this project successfully.”
The results
Schoonover provided leadership and initiative to make this project a success, note people at Dow. It was an excellent challenge to her six-sigma skills. She was tenacious in ensuring the solution was validated and the control plan would truly sustain the project’s goals. She maintained momentum over the two years it took to complete the Measure Analyze Improve Control (MAIC) and capital part of the project. This was a very complex project involving experiment design, lab work, project management and operating expertise, while taking into account the interests of numerous stakeholders of six units.
Rickey Williams, Texas Site optimization and integration leader, says, “The innovation and the multi-manufacturing unit cooperation displayed by this team allowed for a giant step forward in the reliability of the Freeport Site, which is Dow’s largest and most highly integrated site. Good management of a multitude of byproducts from numerous plants is an absolute necessity in the efficient management of a large site. This project not only solved the immediate issue of the corrosion of Stream 3, but the opened the door for more recycle and recovery capability on the site.”
Since its startup in March 2006, the project has boasted essentially defect-free operation and has allowed has allowed for 36 million lbs. of RCL Stream 3 to be recycled instead of incinerated. The more than 17 million lbs. of supplemental HCl feedstock created has yielded approximately $600,000 to $800,000 in cost savings. It was implemented without a single EHS incident. Steve Gluck says, “the R&D team’s technical solution was implemented through a level of operations and personnel complexity that was truly breakthrough.
Indeed, early expectations from stakeholders were very discouraging regarding potential success.” Glenn Lord, R&D Director adds, “Because of the success of this project, we are now evaluating to potentially increase the recycle capacity by incorporating previously excluded wet RCl streams.” Dow’s sustainability goal through eco-efficiency was implemented through creative technical and people solutions.
Bridget was recognized with a Keepsake Award, which is the highest special achievement award given at DOW.
We at Chemical Processing extend our appreciation to our Editorial Board for judging the entries.
Vic Edwards of Aker Kvaener in Houston, Texas
Tim Frank of Dow Chemical Co. in Midland, Mich.
Ben Paterson of Eli Lilly and Company in Indianapolis, Ind.
Roy Sanders of PPG Industries in Lake Charles, La.
Ellen Turner of Eastman Chemical in Kingsport, Tenn.
Ben Weinstein of Proctor & Gamble in West Chester, Ohio
Jon Worstell of Shell Chemical in Houston, Texas
Sheila Yang of Fluor in South San Francisco, Calif.
About the PIA
The Plant Innovation Award (PIA) was inaugurated in 2005 by Chemical Processing to enhance the peer recognition of engineers who solve challenging problems at existing facilities with ingenuity and creativity. Unfortunately, such engineers rarely get visibility beyond their employers for such accomplishments. PIA is the only public award of its kind.
To be considered for the award, the solution must have been successfully implemented at a plant and must have led to significant benefits, e.g., in capacity, quality, safety, environmental compliance, reliability or a combination of factors. Fuller details on the criteria and what’s involved in a nomination are posted on ChemicalProcessing.com.
The CP Editorial Board assesses the innovativeness and significance of the engineering achievements. It can vote to have a single or multiple winners or none at all.
Previous winners of PIA are Praxair in 2006 (see 2006 PAI article) and Dow Chemical and Baxter Healthcare in 2005 (see 2005 PAI article).