Disposable equipment makes lasting gains
About 10 years ago, Tom Warf recalls, Merck & Co. introduced a process that used only disposable equipment at its West Point, Pa., vaccine facility. The combination of disposable filters, tubes, reagent and growth media pouches, and bioreactors (which looked like high-tech resealable bags) yielded 5 liters of hepatitis-A vaccine.
It was the perfect process for disposables, says Warf, an engineer now associated with DME Alliance Inc., an engineering firm in Allentown, Pa. At the time, disposables were best suited for low-volume processes. Because Merck could make the vaccine in such high concentrations and required very little product for each immunization, a single 5-liter bag could produce thousands of doses and was worth approximately $1 million.
“It was also a complex process,” Warf recalls. “The scientist in charge of process development decided it was easier to scale up by using multiples of what he had already done than scale everything up to a larger reactor. We were competing with another company and this let us bring a product to market faster.”
Others had made similar decisions. In 1989, biotechnology pioneer Amgen Inc. was rushing to commercialize erythropoietin, a recombinant hormone to treat anemia. It piloted its process using disposable roller bottles, which researchers had to open constantly to add nutrients and turn to encourage cell growth. Rather than reinvent its groundbreaking production route in stainless steel, Amgen saved time by using a robot to handle thousands of roller bottle operations daily.
Widening use
Since then, disposable equipment has moved into the biotechnology/pharmaceutical mainstream. Only 3% of biopharmaceutical manufacturers use no disposables today, according to the “Third Annual Report on Biopharmaceutical Manufacturing Capacity and Production,” issued in June 2005 by BioPlan Associates, Inc., Rockville, Md..
Filters are the most common disposable. That’s not surprising. After all, such filter capsules have been around for roughly 20 years.
The widest non-filter use involves using disposable bags to add ingredients to a batch or move products between reactors. Yet, fully one-fifth of respondents also uses disposable bioreactors to grow cells or carry out complex reactions.
“Surveys like this often raise more questions than they answer,” says BioPlan president Eric Langer. “Most companies are using dry and pre-filled media and buffer bags, but are they only using them during development or at the pilot stage rather than full production? How many are going to be sold in what capacity?” he asks.
Although Langer plans a follow-up survey, he sees some trends emerging. While no biopharmaceutical manufacturer has replaced existing stainless steel tanks, many have begun to use disposables at certain points in their processes, as well as in process development and pilot plants. Companies racing competitors to market or smaller firms with anxious investors, he says, may opt for disposables to speed commercialization.
Roberta Landon, group product manager for disposable manufacturing at Millipore Corp., Billerica, Mass., agrees. “We’re seeing more and more disposable use in new units to cut the lead time for stainless steel reactor. If you look at the time frame to do all the pipe fitting and welding to fabricate a stainless tank, it takes about six months. Compared with welding, disposable tubing is very forgiving if you need to take off a foot, and that lets you reduce your time frame to about two months.”
Landon says customers are using disposables to prepare media and reagents for existing product lines, taking advantage of disposable convenience without risking active ingredients. Manufacturers also often employ disposables to make drugs for clinical trails rather than invest in expensive stainless reactors prior to regulatory approval. Some multi-product facilities opt for disposables for final fill and finish to reduce the risk of cross-contamination.
The regulatory environment certainly is a major factor in the increasing acceptance of disposables. The U.S. Food & Drug Administration (FDA) must approve every production facility and each process within it. Approval covers everything from materials of construction through validation of each process step through clean-in-place (CIP) procedures after each batch run.
The latter is often a sticking point, says Ted Hutton, a senior business development engineer from polymer supplier Arkema Inc., Wetmore, Colo. “Manufacturers use thousands of gallons of purified water to clean their systems after each batch,” Hutton explains. “They must clean all their tanks and pipes — these are often very large systems — with detergent, then rinse it, then steam it to kill any possible living organisms. They do this continuously throughout the day and must then properly dispose of the water. With disposables, they can buy pre-validated bags, tubing, and valves and eliminate some of the steaming.”
A typical turnaround takes a full day or even longer. By using pre-validated disposables to reduce cleaning time, companies can boost productivity and increase effective capacity while complying with FDA guidelines, he says. Also, because bags take less space to store than drums or other containers, they free up plant floor space for more productive uses.
Economics
Proponents claim disposables are economical, even though they can be expensive. For example, disposable bioreactors, which consist of multilayered bags and usually include mixing systems, sampling ports, integral tubing and instrument probes, typically cost $200 to $300 each.
Landon, who often must make an economic case for disposables, notes, “First, you have to look at the high upfront capital investment in stainless compared with the very small investment in consumables, though the cost of consumables goes up over the life of the product.” She cautions, “A lot of existing stainless steel processes use more consumable items than a lot of customers realize. There’s water usage, purification costs, utilities. If you use caustics and chemicals for CIP, how do you dispose of those chemicals? You have to neutralize them and some facilities must then pump them out or cart them away.”
Even then, speed often trumps conventional economics. “Time to market is critical if you want to take advantage of your patent,” says Warf. “If you’re making $1 on every penny investment, the difference between 3¢ for stainless and 5¢ for disposables is nothing.”
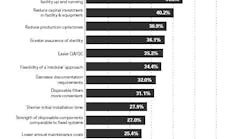
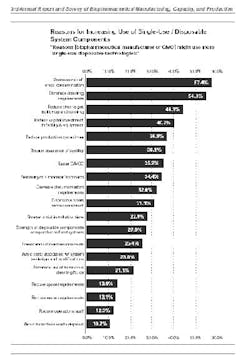
In addition to speed, cost, and reduction in cleaning requirements, manufacturers also chose disposables to decrease the risk of cross-contamination and enhance sterility and quality assurance (see chart).
Reasons for increasing use of single-use system components (click image to enlarge)
Pitfalls and promise
Of course, disposables pose their own concerns. Plastics are prone to ripping and puncture. Each inlet and outlet is a potential source of leaks. Reagent bags are often frozen to keep air from permeating and reacting with their contents, but they may crack if defrosted too quickly. All but the smallest bags require some sort of support. Also, attaching disposable tubes to other equipment while maintaining their sterility is no simple matter, says Charles Meadows, a sales engineer at disposables producer Mitos Technologies, Inc., Phoenixville, Pa.
BioPlan’s survey showed that more than half of companies had restricted disposables’ use because of concerns over bag breakage and lost production. Even more users worried about extractables, unreacted monomers and additives that could leach out of plastic containers to contaminate products.
“Interactions between plastic materials and pharmaceutical products are quite real, and they may include sorption or leaching,” says Michelle M. Gonzalez, a principal corporate engineer at Amgen, Thousand Oaks, Calif.
Such issues have generally limited disposables to simple or low-volume applications. Tubing and bags for storing and dispensing buffers, reagents, and, more recently, growth media are well established, says James Vogel, associate director of site engineering for Amgen’s West Greenwich, R.I., facility. Gonzalez sees growing use in bags for sampling and dispensing bulk harvest fluid and intermediate/final products. Some disposables are even used in blending and formulation.
Bioreactors rank as the most advanced disposable application — and the least common. “The available technology and sizes are not large enough at 250 liters to address commercial-scale manufacturing,” says Gonzalez.
Biotech companies most commonly rely on bioreactors in “seed trains,” where they grow cell lines to express proteins used in genetically modified drugs, says David Marks, president of DME Alliance. “You can’t just dump everything into one reactor,” he explains. “The interactions cells have with each other require a certain density. Instead, you start with an inoculant, grow it to a certain cell density, and then use it as an inoculant for a larger bioreactor.”
Disposable bioreactors are well suited to such small-scale work, says Marks, but run into issues with mass transfer, mixing, and bag handling as they grow larger.
Several disposables’ manufacturers are attempting to overcome that obstacle. Stedim S.A. of Aubagne, France, the acknowledged disposables market leader, provides integral impellers for bags of up to 1,000 liters, though it recommends recirculation mixing with a peristaltic pump for smaller bioreactors. Others have turned to unique technologies (see sidebar).
Despite the swell of innovations, disposable bioreactors face an uphill battle. “Any regulated life sciences industry is going to be very conservative,” says Marks. “If you’re doing something novel, the burden of proof is on you to prove that it won’t affect product or public safety.”
Beyond biopharmaceuticals
Some industries, such as adhesives, use disposable mixers. However, only a few manufacturers make products valuable and sensitive enough to justify disposable bags or tubes.
The semiconductor industry is one area where disposables have taken hold. Producers of oxygen-sensitive photolithography chemicals often make and ship them in disposable bags, says Reinhard Hanselka, a principal chemical engineer at Integrated Engineering Services, Inc., Santa Clara, Calif. Shipping the chemicals in the same bags they are made in reduces the risk of oxidation, plus semiconductor makers find the used bags easier to dispose of than glass.
Marks sees potential applications with other oxygen-sensitive chemicals and in nanotechnology.
One drag on the potential growth of disposables is not technical in nature. Instead, it stems from the technology’s rapid but uneven growth. Most disposables are customized for specific applications and there are few standards.
“There is no standard testing criteria,” says Vogel. “The key issue is chemical interactions. Each application has its own requirements and/or limitations, and the vendors do not have a uniform method to show compatibilities.” Moreover, adds Hutton, “FDA won’t let you change from one manufacturer of disposables to another without revalidating.”
That may change soon. ASME’s Bioprocessing Equipment Standards Committee plans to address materials and product testing issues in future editions. Hutton, who is chair of the Polymers Subcommittee, says it hopes to issue a new standard covering tubes and bags as soon as 2007 that would address everything from permeation and extractables to physical properties.
This would allow manufacturers for the first time to compare one vendor’s offerings with another’s. It would also make it easier for FDA to approve process changes as technology continues to advance.
It may lead to a new type of biopharmaceutical manufacturing. “Interchangeable disposables could make whole systems a lot more versatile,” says Hutton. “If you have to tweak a process, maybe you’ll be able to alter the line without revalidating everything.”
Equipment advances
Vendors of disposables ranging from filters to mixing systems are striving to make units more effective and affordable.
Bioreactors have drawn many innovators. One of the best known, Wave Biotech, LLC, Somerset, N.J., employs a mixing system that involves placing a bag filled with fluid on a rocker panel and inflating it with air until it becomes stiff. As the panel rocks the bag back and forth, the wavelike motion of the liquid mixes and aerates the ingredients. Wave is working on adding baffles to improve agitation.
LevTech, Inc., Lexington, Ky., uses superconductors mounted on a revolving cassette to levitate and turn a single-use impeller inside a disposable bioreactor mounted above. With no shafts, bearings or seals, the system minimizes the risk of leaks and tears. A single motor is capable of mixing reactors ranging from 20 to 2,000 liters containing fluids with viscosities up to 800 centipoise.
Meanwhile, Frederica, Del.-based ILC Dover LP offers a mixer that works like an accordion. It consists of a perforated diaphragm attached inside a plastic mixing bellows. As an external mechanism moves the disposable bellows up and down, the fluid moves through the diaphragm, mixing without impellers or actuators. The system is scalable to 10,000 liters.
Because most media and reagent bags come pre-sterilized, users cannot easily modify them while retaining their sterility. Not so with the new line of Klave-it bags from Mitos Technologies. “This is made from Kynar polyvinylidene fluoride and is the first bag that can withstand autoclaving,” says sales engineer Meadows. “You can change the setup of your probes or tubes and then sterilize the bag again. It also has enough chemical resistance to use with aggressive buffers and cleaning solutions.”
Even such well-established disposables as filters continue to evolve. The new Pod platform from Millipore Corp. is an expandable depth filter. Its three disposable filters have 0.1, 0.5, and 1.0 m² of filtration area. The pilot-scale Pod holder accommodates from one to five 0.1-m² filters. The process-scale unit holds from five to 30 1.0-m² filters. Users adjust the depth of the filter to their requirements in normal flow clarification and pre-filtration applications.