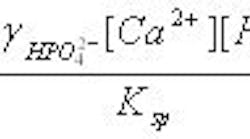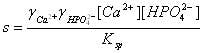Treat precipitation reactions as reactive crystallizations
In disposing of phosphoric acid waste, simply neutralizing the waste with caustic and sending it to a wastewater treatment plant (WWTP) often is not an option because the resulting phosphate salts are soluble in water and the total amount of phosphate that can be discharged from the WWTP is strictly controlled for environmental reasons. Incineration methods are sometimes used, but they can be expensive and generally do not allow recovery of the phosphate content.
A number of years ago we implemented an alternative method involving reaction of phosphoric acid with hydrated or “slaked” lime (Ca(OH)2 particles slurried in water) to precipitate calcium phosphate solids. The processing scheme involved adding slaked lime to a batch of phosphoric acid in a stirred tank, and filtration of calcium phosphate solids from the resulting slurry; we opted for batch operation as it fit well into the specialty chemicals plant where the phosphoric acid waste was generated. Because calcium phosphate solids are only sparingly soluble in water at neutral pH, the filtrate from this process could be sent to the WWTP. The low product solubility not only allowed removal of more than 95% of the phosphate present in the feed, but also made the task of building large, filterable particles challenging. This article explains why and illustrates the importance of understanding and controlling solute supersaturation levels in a crystallization operation.
Chemistry and product solubility
The mix of calcium phosphate reaction products generated depends upon the ion speciation — that is, the type of ions present in solution, whether Ca2+, H2PO4-, HPO42-, or PO43-, among others. This depends upon pH and also upon whether the products of the various possible calcium phosphate reactions are fairly soluble and able to undergo further reaction in solution, or only sparingly soluble. A review of the literature [1, 2] indicates that hydroxyapatite, Ca10 (PO4)6(OH)2, is the primary product obtained at alkaline conditions, while dicalcium phosphate dihydrate, CaHPO4•2H2O, is the primary product at pH 2-4 and temperatures below 80°C. We focused our efforts on crystallizing CaHPO4•2H2O. Understanding the reaction product’s solubility behavior in the mother liquor is critical to understanding supersaturation, the driving force for crystallization. Figure 1 illustrates the solubility/dissociation behavior of CaHPO4•2H2O in water at pH 2-5 and 37°C, as estimated from solubility isotherms reported by Elliott [2]. Solubility decreases rapidly above a pH of about 2.5. This corresponds to the pKd value of the dissociation constant for that compound. At pH 7, the solubility of CaHPO4•2H2O is about 150 ppm.
Promoting crystal growth
The relationship between nucleation and growth provides the basis for understanding the conditions needed to promote crystal growth. For dicalcium phosphate, the supersaturation level can be expressed as:
where g denotes the activity coefficients for the ions in solution, [Ca2+] and [HPO42-] are the concentrations of ions in the supersaturated solution, and Ksp is the solubility product, which equals [Ca2+][HPO42-] at equilibrium at the given solution conditions. The numerator is the ionic activity in the supersaturated solution and the denominator represents thermodynamic equilibrium.
The nucleation rate often is defined as the number of crystals produced per unit time per unit volume of crystallizer. It generally follows a power-law relationship such that Nucleation Rate µsn(2).
The rate of crystal growth, often expressed as a change in a characteristic crystal dimension per unit time, is generally a more-linear function of supersaturation. For this reason, the growth of existing crystals will dominate nucleation at supersaturation levels below a certain critical level [3-6].
General control strategy. For the purpose of producing large, easily filtered crystals, control of a batch crystallization operation can be viewed in terms of initiating a nucleation event followed by controlling a growth period. The control strategy involves manipulating the operating variables that affect the rate at which supersaturation is generated — so as to first nucleate a small population of crystals and then grow those crystals with minimal additional nucleation. The approach to building large crystals is also relevant to producing higher purity crystals. How a given crystallization process is operated to generate supersaturation will depend upon the particular application and may include slow change in temperature, evaporation of solvent, addition of an anti-solvent, or reaction of reagents to generate a product concentration in excess of the product’s solubility in the mother liquor, as in the present example. After nucleation, supersaturation should be kept at a level that minimizes or at least reduces to an acceptable extent the nucleation of additional crystals.
Techniques to avoid high supersaturation. In carrying out precipitation reactions, special care is needed to limit as much as possible the generation of high localized supersaturation levels at the reagent feed point. In the example, the base is added to the reactor as a slurry of Ca(OH)2 particles suspended in water, so Ca(OH)2 particle size, the available solid surface area, and mass transfer to and from the solid surface — as well as the chemical kinetics and product solubility — play a role in generating supersaturation. (For discussions of the general kinetic aspects of solid/liquid reactive crystallization processes, see Refs. 7 and 8.) In general, the supersaturation level at the feed point may be minimized by improving circulation (without generating too much shear), using dilute reagents, slowing down the reagent addition rate, and operating under conditions that increase product solubility.
Developing an operating scheme
The following guidelines are useful for determining a satisfactory scheme: "
Start at conditions of high product solubility. In the application, the solubility of the reaction product is reasonably high at pH 2 (Figure 1). Relatively large crystals of CaHPO4•2H2O can be grown in a batch reaction by mixing the phosphoric acid and slaked lime in the proper proportions for pH 2, allowing sufficient time for everything to go into solution, and then slowly adding slaked lime until solids begin to form. In this way, nucleation of crystals can be made to occur near saturation conditions— that is, at a reasonably low supersaturation level. A low level of supersaturation can be maintained by continuing slow addition of Ca(OH)2, gradually increasing the pH. This approach allows the system to stay close to the saturation conditions illustrated in Figure 1, thereby promoting growth of existing CaHPO4•2H2O crystals and avoiding rapid nucleation of new ones. In this case, adding the base over a period of about one hour yielded a filter cake consisting of rod-shaped crystals as large as 20 microns in diameter by 100 microns long (Figure 2), plus many smaller crystals on the order of 10 microns in length.
The example thus involves careful negotiation of a steep pH-dependent solubility curve and, in this respect, resembles crystallization of organic acids and bases from aqueous solution by adjustment of pH across the pKa value [9] to form the non-ionized species. For example, we are familiar with an industrial crystallization process involving the addition of an inorganic base to an aqueous solution containing an ionized organic compound with amine functionality (R-NH3+). Addition of base deprotonates the ionized species to crystallize the free base form (R-NH2) as pH is raised above the pKa for that compound. Another example involves crystallization of organic acids from an aqueous solution by slow addition of a mineral acid [10]. In that case, the ionized form of the organic acid (R-COO-) is protonated as the pH is reduced below the pKa value, and the protonated form (R-COOH) crystallizes from solution.
Avoid starting at conditions of low product solubility. Approaching the precipitation from basic conditions by adding acid to a pool of slaked lime forced rapid nucleation. This is because hydroxylapatite, the product obtained at alkaline conditions, is essentially insoluble. Because product solubility was so low, the level of supersaturation was always high, even if acid was added slowly. As a result, nucleation dominated growth. We could not grow particles any larger than about 10 microns in size (Figure 3), and subsequent filtration was slow. Comparing Figures 2 and 3 provides a dramatic illustration of the impact that supersaturation level can have on crystal size.
Scale-up considerations
To succeed at commercial scale, it is crucial to take three factors into account: Cycle time adjustments. In scaling up a batch processing scheme developed in the laboratory, adjustments in cycle time may be necessary. This is because mixing quality generally declines on scale up, and a decrease in mixing quality can cause an increase in localized supersaturation levels in the vessel. As mentioned earlier (and worth emphasizing), slowing down the base addition rate (or diluting the base) may help mitigate this effect. Slow addition of reagent is most important at the beginning of the crystallization; the addition rate may be increased toward the end of the batch for increased productivity.
Mixing and heat transfer effects. It is also important to note that performance at the commercial scale can vary significantly depending upon the mixing and heat transfer capabilities of the equipment selected for the operation. Often it makes sense to use an existing vessel to minimize installation costs — however, it is essential to ensure that reasonably good performance can be achieved. For example, mixing impellers should be selected to provide good axial-flow circulation within the vessel with minimal shear. Retrofitting an existing vessel with a more-efficient impeller (or set of impellers) can provide substantial improvement in crystallizer performance. For a glass-lined vessel with a retreat-blade or “crowfoot” impeller, consider a more-efficient axial-flow impeller as a replacement. Also consider adding baffles to the tank for improved mixing performance. (Consult the vessel manufacturer to explore available options.) Although retrofitting a new impeller or baffles into a glass-lined vessel may be a difficult proposition, doing so can yield significant improvements in crystallizer performance. Replacing an existing pitched-blade impeller with a more-efficient and properly-sized hydrofoil design may also boost performance depending upon the application. In addition, we have found it useful to specify a variable-speed motor or mechanical drive – to allow adjustment of impeller rotational speed on startup in the plant. This provides some added flexibility for optimizing the operation in the field. (For more detailed discussions of mixing considerations, consult Refs. 3-5.)
Heat transfer is particularly important in the present example because of the highly exothermic reaction. A pump-around heat exchanger sometimes is used to improve heat transfer capability instead of relying on a heat transfer jacket alone. However, keep in mind that the circulation pump should be selected to avoid generating too much shear; excessive shear can result in unwanted crystal nucleation as well as crystal attrition or breakage. Vendors offer a number of low-shear pump designs as alternatives to standard centrifugal-pump designs.
Feed pipe location. For the present application as well as similar reactive crystallizations, we adding reagent to the crystallizer vessel through a dip pipe located below the liquid level – to facilitate rapid distribution of reagent throughout the vessel during crystallization. Locating the outlet of the feed pipe near the suction side of an axial-flow impeller works well. This can yield much better results than simply pumping reagent into the vessel through the head space on top of the liquid. In our application, we installed only a base-addition dip pipe. An acid-addition dip pipe is not needed in this case.
Acknowledgments
The authors thank Joseph Bonadies, Jr., Ray Collins, Sumnesh Gupta, Kishore Kar and Tracy Kiefer for many valuable discussions.
References
1. Gard, D.R., “Phosphoric Acids and Phosphates,” Volume 18 in “Kirk-Othmer Encyclopedia of Science and Technology,” 4th Ed., J.I. Kroschwitz and M. Howe-Grant, eds., John Wiley & Sons, New York, pp. 669-718 (1996).
2. Elliot, J.C., “Structure and Chemistry of the Apatites and Other Calcium Orthophosphates,” Elsevier, Amsterdam (1994).
3. Genck, W.J., “Crystal Clear – Part 1,” Chemical Processing, Vol. 66 (10), pp. 63-67 (Oct. 2003).
4. Genck, W.J., “Crystal Clear – Part 2,” Chemical Processing, Vol. 66 (12), pp. 37-42 (Dec. 2003).
5. “Handbook of Industrial Crystallization,” 2nd Ed., A.S. Myerson, ed., Butterworth-Heinemann, Boston (2002).
6. Tadayyon, A., S.M. Arifuzzaman and S. Rohani, “Reactive Crystallization of Brushite Under Steady State and Transient Conditions: Modeling and Experiment,” Ind. Eng. Chem. Res., Vol. 42, pp. 6,774-6,785 (2003).
7. Kelkar, V.V. and K.M. Ng, “Design of Reactive Crystallization Systems Incorporating Kinetics and Mass-Transfer Effects,” A.I.Ch.E. J., Vol. 45(1), pp. 69-81 (1999).
8. Bechtloff, B., P. Juesten and J. Ulrich, “The Kinetics of Heterogeneous Solid-Liquid Reaction Crystallizations – An Overview and Examples,” Chemie Ingenieur Technik, Vol. 73(5), pp. 453-460 (2001).
9. Butler, J.N., “Ionic Equilibrium: Solubility and pH Calculations,” John Wiley & Sons, New York (1998).
10. Wang, F. and K.A. Berglund, “Monitoring pH Swing Crystallization of Nicotinic Acid by the Use of Attenuated Total Reflection Fourier Transform Infrared Spectrometry,” Ind. Eng. Chem. Res., Vol. 39, pp. 2,101-2,104 (2000).
Tim Frank is a research scientist and senior technical leader in the Engineering and Process Sciences Laboratory at The Dow Chemical Company, Midland, Mich. E-mail him at [email protected].
Wayne Fort is a senior research engineer in the Agricultural Chemicals Technology Center at The Dow Chemical Company, Midland, Mich. E-mail him at [email protected].
Chris Jones is a senior research engineer in the Engineering and Process Sciences Laboratory at The Dow Chemical Company, Midland, Mich. E-mail him at [email protected].