Even if no one has ever suggested that your plant has a mercury problem, it is worthwhile to think carefully about whether the wastewater's mercury level is truly acceptable.
During the mid-1990s at one large facility, an indirect discharger on one of the Great Lakes, plant effluent mercury levels appeared to be reasonably low. Analytical data showed mercury concentrations of 1 microgram/liter (g/l), or 1 part per billion (ppb), at worst, and usually less.
This effluent concentration would be acceptable in most sanitary districts and is lower than the 2 g/l of mercury allowable in drinking water. However, because the wastewater flow from the plant is so great ," accounting for approximately one-third of the host town's wastewater flow ," and because the Great Lakes ecosystem is so sensitive, the local wastewater authority encouraged an investigation into the root cause of the mercury concentrations.
A careful examination of the plant's feedstocks revealed that the plant's sulfuric acid was obtained from a lead smelter and was contaminated with as much as 10,000 g/l of mercury. By switching to an alternative source of commercial sulfuric acid, the plant achieved a 98 percent reduction in its effluent mercury level. The wastewater authority now holds up the plant as an example of success under the authority's current pretreatment ordinance limit of 0.3 g/l and its policy goal of zero discharge.
This example underscores the importance of fundamentally investigating and understanding the mercury concentrations in a plant's wastewater. Before considering the practical issues at an individual plant, however, it is helpful for chemical and pharmaceutical plant personnel to review current mercury regulations and to develop an understanding of the mercury problem.
With that context in place, it then is appropriate for plant personnel to discuss a proactive approach for addressing the concern before it becomes a regulatory problem. The most appropriate approach usually is one that emphasizes careful materials management, giving secondary attention to end-of-pipe treatment.
Rethinking regulations
Why is mercury in wastewater such a problem, and why is it more likely to arouse regulatory scrutiny now than it did in years past? Appreciation of chemistry and toxicology and improvements in laboratory techniques all are driving mercury to a higher profile and pushing wastewater standards to tougher levels.
The chemical fate of mercury in nature truly is unfortunate. Most other water pollutants become diluted in the receiving water and then become assimilated through natural processes that gradually render the pollutants harmless.
For example, if dissolved hydrocarbons are present at acceptable levels in an industrial effluent, they quickly become diluted to a lower concentration in the receiving water and then become consumed as a food source by microorganisms. Through the action of the microorganisms, the hydrocarbons are broken down into carbon dioxide and water. The combination of dispersion and biological action, therefore, eliminates the hydrocarbons from the environment.
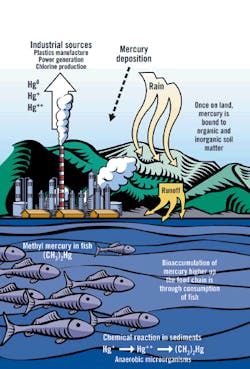
Mercury is different. Unlike most other pollutants, mercury is both persistent and bioaccumulative. Because mercury is an element, it cannot be broken down into innocuous components. In fact, microorganisms convert dissolved mercury into methylmercury, an organic compound that is more active and toxic biologically than is the original metal.
Once methylmercury enters the food chain, it bioaccumulates and bioconcentrates. As a result, the most contaminated fish tissues tend to be in larger, older predator fish ," precisely the fish most valued for human consumption. Game fish tissues easily can contain several million times the mercury levels of their native water. Furthermore, the methylmercury is bound into the flesh of the fish; therefore, gutting, skinning and cooking the fish provide no benefit in reducing the mercury content.
Advances in the understanding of mercury's human toxicological properties also have focused attention on the metal. The general dangers of mercury have been known for centuries. "Mad as a hatter" was a popular expression arising from the odd behavior of hat makers exposed to mercury fumes.
In the early 1950s, mercury first emerged as a wastewater-related health concern when a Japanese chemical plant discharged mercury-laden effluent into Minamata Bay. Fish comprised a large portion of the local diet, so many of the residents of Minamata City became ill with mercury poisoning. They experienced a loss of peripheral vision, a pins-and-needles sensation in their hands and feet, a loss of coordination and difficulty speaking, hearing and walking.
The United States has avoided such notorious scenarios of mass poisoning by mercury, but the nation's waters contain numerous fish that are unsafe to eat without restriction. Fish advisories have steadily increased over the years ," in 2000 alone, 41 states issued fish advisories.
To control mercury in natural waters before the element has an opportunity to contaminate fish tissue, the U.S. Environmental Protection Agency (EPA) established a number of controls such as ambient water criteria. Under the National Toxics Rule, promulgated by EPA in 1992, the ambient criterion for mercury is 12 nanograms per liter (ng/l), or 12 parts per trillion (ppt). In the Great Lakes system, the ambient criterion is even lower ," just 1.3 ng/L under EPA's Great Lakes Water Quality Initiative.
And certain watersheds ," designated by states as impaired waterbodies ," are regulated under EPA's Total Maximum Daily Load (TMDL) program. Established under Section 303(d) of the Clean Water Act, the TMDL program requires states to specify how much a particular pollutant needs to be reduced for a water body to meet water quality standards. The state then allocates pollutant load reductions among a watershed's pollutant sources. In practical effect, the TMDL program easily can dictate ppt discharge concentrations because of mercury's tendency to bioaccululate in fish.
Regional regulatory bodies also regulate mercury. In the Great Lakes basin, for example, the International Joint Commission recommended zero discharge as the goal for Lake Superior.
Local wastewater authorities also are imposing increasingly strict mercury standards to protect the quality of their wastewater treatment plants' sludge, which treatment plants often use or sell in a processed form as fertilizer. If mercury levels are too high, the wastewater treatment plant might need to pay for disposal of its otherwise-useful sludge as solid waste.
Detection improvements
Advances in laboratory technique also are responsible for the current interest in mercury. Until recently, the lowest detection limit for mercury using an EPA-approved method was 0.2 g/l, using Method 245.1, which was issued in 1974. Its detection limit is one-fifth of ppb; therefore, any regulatory concern expressed as ppt was a moot point because it simply was not measurable.
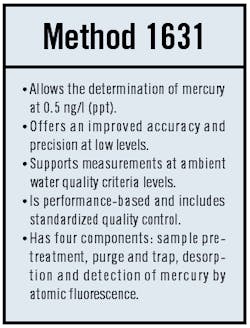
The situation changed with the issuance of Method 1631 in 1999. Under Method 1631, a minimum level of 0.5 ng/l is achievable ," 400 times lower than the previous lowest detection limit. As a result, it is now possible, in a practical sense, to assess compliance with regulatory standards set at the ppt level.
Although most pretreatment permits currently held by industry are written at the ppb level and rely on Method 245.1 for measurement, the situation could change gradually. First guidance, then ordinances and, finally, pretreatment permits could be reissued to reflect the new lower level of achievable resolution for at least some of the mercury measurements.
Revealing hidden mercury
We expect to see mercury in products such as batteries, fluorescent bulbs, silent switches, thermostats, dental amalgams, preservatives such as thimerosal, preserved solutions and old fungicide stockpiles. In each of those products, the mercury plays a clearly defined role, so its presence is not remarkable.
With the arrival of better laboratory techniques, however, comes a troubling revelation: Traces of mercury are widespread in other industrial chemicals and commercial products. In fact, many products with no apparent connection to mercury can contain significant traces of the metal.
Commercial-grade chemicals produced in the mercury-cell process are particularly noteworthy sources of inadvertent mercury. Sodium hydroxide (caustic soda), potassium hydroxide, chlorine and hydrochloric acid (muriatic acid) all can be produced in the mercury-cell process.
Another example of a commercial-grade chemical frequently containing inadvertent mercury is sulfuric acid produced at lead and copper smelters.
Direct users of these mercury-contaminated industrial chemicals can be using and discharging mercury inadvertently. The same is true for indirect users of these chemicals because the chemicals are incorporated into commercial products.
Many commercial products contain traces of mercury. Soaps, chlorine bleaches, detergents, fixatives and reagents all potentially can be contaminated with traces of mercury.
A Massachusetts database ," the MASCO database ," listing 5,508 products used in hospitals states that 781 contain measurable traces of mercury. Of those products, 469 have concentrations above 10 g/l, meaning almost 10 percent of the tested hospital products contain significant mercury concentrations.
Fuels such as coal and, to a lesser extent, oil generally contain traces of mercury. And wet scrubbers can convert mercury air emissions into mercury-laden wastewater.
Considering all the potential sources of hidden mercury, it is not surprising that more and more facilities are discovering they have a mercury problem. Fortunately, it is relatively easy to diagnose whether or not your plant has a mercury problem and to take protective measures to address the issue.
Making the diagnosis
A review of historic wastewater analyses can provide a rough initial mercury assessment, but it is only a starting point because of the limitations associated with these analyses.
A review of Material Safety Data Sheets (MSDSs) for every chemical product coming into the plant can provide some additional information. The trace-elemental composition of the fuels used at the plant also should be considered.
A significant limitation of MSDSs is they generally do not identify impurities composing less than 1 percent of the product. As a result, a product could contain as much as 10,000 milligrams/liter (mg/l) of mercury and still not disclose the presence of mercury in its associated MSDS.
A more reliable way to learn the mercury content of a chemical is to ask the supplier for a certificate of mercury analysis. A certified report of mercury concentration should be expressed in ppb. If the supplier's certified report claims "no mercury," then the report should reveal the detection limit of the test that was used to establish the claim.
By asking for a certified report of mercury concentration, plant personnel will be able to develop data vastly better than what are available in the MSDSs. Of course, it also is possible to send samples of your plant's incoming chemicals for analysis, but such analyses quickly can become quite expensive if more than a few chemicals are involved.
Remember: All mercury entering a plant must exit somehow. Therefore, as a first cut, it is safe to assume the mercury leaves the site in the wastewater. Depending on the complexity of your plant and the number of mercury-containing materials, it might be useful to track the pathways of mercury in the plant using a flow chart of the processes.
An ounce of prevention
The best approach to mercury problems is usually one that emphasizes prevention instead of treatment. As an element, mercury cannot be destroyed, only shifted from one medium to another.
To trade a mercury-contaminated wastewater for a mercury-contaminated sludge, ash or air emission is not really satisfactory, even if it satisfies a wastewater regulation. In addition, prevention usually costs less than treatment. So if it is at all possible, address the mercury problem at the source.
Consider adding a mercury-content criterion to feedstock specifications. A mercury criterion can disqualify contaminated materials and encourage your vendors to adjust their formulations to reduce or eliminate mercury. If the mercury content in the plant's fuels is significant, consider a mercury-content fuel specification ," or the substitution of alternative energy sources.
To address the problem of mercury that is "deliberately" present in certain feedstocks, more research and creativity are required because the properties of mercury are presumably vital to the product's performance. For deliberate mercury formulations, it is worthwhile to consider whether mercury-free alternatives are available.
Sometimes a literature review will reveal mercury-free alternatives that others have identified. Other times it will be necessary to be a leader and develop new substitutes for the offending material. The research and development required for alternatives takes time, so it is better to begin the work long before a regulation or pretreatment standard forces the issue.
Sources and Paths of Mercury in the Environment
Source: EPA, "Mercury Transport and Fate in Watersheds," 2000
Resorting to treatment
If mercury cannot be eliminated satisfactorily from the process, it might be necessary to resort to wastewater treatment. A variety of wastewater treatment processes currently are available.
Filtration can be considered if the mercury is attached to suspended solids or colloids. A well-designed simple filtration unit can remove particles down to 5 microns. If necessary, membrane filtration can remove mercury to the sub-micron size.
Reverse osmosis (RO), like membrane filtration, also can provide submicron removal of colloidal mercury. RO systems are fouled easily; therefore, the RO system usually is reserved for a polishing step at the tail end of a treatment train in a waste stream of any complexity.
Ion exchange can be considered as a treatment option if the mercury is present as dissolved ions in the wastewater. Ion-exchange resins can achieve near total deionization of wastewater. In wastewater applications, the expensive ion-exchange resins are fouled easily. If the waste stream is complex, therefore, it usually is necessary to treat the wastewater's gross contaminants first and to use the ion exchange as a final polishing step.
Selective polymeric adsorption also can be considered if the mercury is present in ionic form. Selective adsorption is attractive if, for example, mercury (II) is present in the wastewater with other +2 charged ions. Some selective polymers can be reused, minimizing the production of secondary sludge.
Activated carbon adsorption can provide effective removal of mercury at the ppb level. Organic mercury is adsorbed readily, and various specialty activated carbons are available for removing metallic and ionic mercury as well.
Oxidation/reduction followed by precipitation also is an option. However, the redox reactions are not selective for mercury, so they will result in a sludge that contains a variety of metals, not just mercury.
As long as the appropriate climate, space and geology are available, constructed wetlands can remove mercury from wastewater. Care must be taken in the design and testing of the wetlands to ensure the mercury is captured in the wetlands. It also is important to verify that the mercury is not converted into methylmercury, which would be counterproductive.
Any one of these treatment technologies can be expected to produce better results at a lower cost if it is placed close to the point of mercury generation. Mercury treatment near the source minimizes the volume and complexity of the wastewater stream.
Conclusion
As ordinances and laboratory practices in the field catch up with the state-of-the-art laboratory detection methods and the national movement to restrict mercury, mercury-containing wastewater is destined to become a more important issue. The current period of transition provides a fine opportunity to diagnose and solve your own plant's latent mercury problem before it becomes a regulatory problem. CP
Gilbertsen is a program manager for ENSR International, Warrenville, Ill. Contact him at (630) 836-1700.