Whether isolating a substance of commercial interest in the laboratory, purifying a drug or treating wastewater, separation technologies remain critical to the chemical process industry. The separations market continues to grow, driven by existing and new needs for wastewater, biotechnology, pharmaceutical and petrochemicals applications.
In fact, the market for all advanced novel separations techniques is expected to grow at an average annual rate of 23.4 percent until 2005, according to Business Communications Co., a Norwalk, Conn.-based market research firm. Membranes ," thin-film barriers that allow preferential passage of certain substances ," are leading this growth. These permeable barriers provide functional transport across the barrier and are characterized by properties of selectivity and flux.
This article focuses on membrane applications and takes a look at how these technologies are impacting chemical processes today, as well as how they might impact these processes tomorrow.
Taking center stage
The chemical industry does not bear witness to radical change often. The "next big thing" is seldom developed and implemented overnight. Many months and even years can lapse from the start of a research and development (R&D) project to widespread commercialization. Even then, industry perception can act as an additional barrier to technology adoption.
Take a look at membranes, for example. Membrane technology is not new. It has been commercially available for more than 30 years. However, membranes often have been relegated to secondary operations such as water purification upstream in the pharmaceutical industry, and to waste treatment or pollution control downstream. Today, however, they increasingly are being used or considered for mainstream operations.
The rising adoption of membrane separation technologies largely has been driven by the increasingly strict environmental regulations enacted over the last several decades, according to Membrane Separation Technologies to 2006, a new report from The Freedonia Group, Cleveland.
Some of these regulations include the U.S. Environmental Protection Agency's (EPA) Clean Water Act, Safe Drinking Water Act, Surface Water Treatment Rule and new arsenic rule, as well as food and drug purity rules from the U.S. Food and Drug Administration (FDA), according to Jennifer Mapes, the Freedonia industry analyst behind the report.
On demand
Today's markets for membranes all have one very specific requirement in common ," purity. These markets increasingly are turning to membranes because conventional filtration processes just do not provide the necessary level of purity, says Mapes.
However, Steve Matson, Ph.D., of the Worcester Polytechnic Institute (WPI) in Worcester, Mass., acknowledges that membranes alone are not "particularly good or efficient at achieving very high product stream purities." Instead, they are better suited to effecting bulk separations.
"As a result," he explains, "they can be paired with other types of separations in so-called hybrid' processes where a membrane accomplish[es] the bulk of the separation task and another separation process like adsorption accomplish[es] the last bit of the separation to achieve high product purity."
Although membranes are gaining acceptance in many industrial processing areas, they still have a ways to go in others.
"Membranes are not widely applied today in commodity industrial chemical processes for a variety of reasons," says Dr. Dickson E. Ozokwelu, lead technology manager (chemicals, petroleum and forest products) at the U.S. Department of Energy's Office of Industrial Technologies (OIT). "The foremost reasons are the cost of the membrane systems and the [long] lifetimes of [other] commercially viable technologies."
Energy savings
Membranes have been explored as an alternative to distillation because of their potential to dramatically reduce energy and manufacturing costs. Distillation remains the most widely used separation technology in the chemical industry, says Ozokwelu. However, this process heats the fluids to boiling, consuming a large amount of energy.
And the need for energy-efficient processes has never been greater, especially in petroleum refining. In 1994, the U.S. petroleum industry used 6.3 quadrillion British thermal units (Btu), making it the nation's most energy-intensive industry, according to OIT. Within the refining industry, separation processes ," primarily distillation ," account for nearly 40 percent of energy use.
A current OIT project is focusing on the use of membranes as a replacement for distillation in petroleum refining. Unlike distillation, membrane separation requires no heat. Instead, it relies on differences in the rate at which components pass through the membrane material.
The project, says OIT, will center on pervaporation separation of hydrocarbon mixtures and the use of reverse-selectivity membranes for hydrogen recovery. With pervaporation, the liquid phase contacts one side of the membrane, and the permeate side contacts a reduced-pressure gas phase. Because membranes undergo physical and chemical changes when exposed to organic liquids and elevated temperatures, researchers must find a way to develop pervaporation membranes that can withstand harsh operating conditions.
Two inorganic membranes are being considered for the project ," polymer membranes for the hydrocarbon separations and ceramic membranes for the hydrogen separations. Both types of materials have inherent shortcomings for membrane applications, says Ozokwelu, and a lot of hurdles remain.
"More progress has probably been made with the polymer membrane for hydrocarbon separations than with [the] ceramic membrane for hydrogen separations," he adds. "Hydrogen separations from mixtures using membranes have been a notorious challenge, and industrial investigations have been going on for 60 years. But the return for successful technology would be immense."
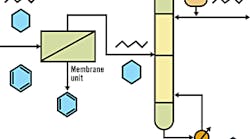
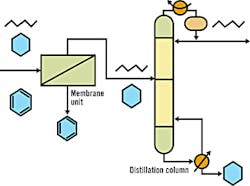
Membrane/Distillation Hybrid Process to Separate Aromatic and Aliphatic Hydrocarbons
Source: DOE's Office of Industrial Technologies
Membrane materials
Polymeric membranes continue to dominate the membrane market, both in terms of value and volume, states the Freedonia report.
"This is a huge category of products that account for the vast majority of membrane demand," says Freedonia's Mapes. "In general, they are suitable for just about any application, with the specific selection depending on the components of the feed stream, the operating pressure of the system, the flux rate desired and the use of chemical-based pre-treatments."
When conditions get rough, however, nonpolymeric membranes are becoming the choice of many processors. In fact, says the Freedonia report, demand for nonpolymeric materials, including ceramic, metal and composite types, will record more rapid gains through 2006, particularly in certain specialty applications. These applications include pharmaceutical processing, gas separations, petroleum refining, hazardous waste treatment, fine chemical production and other applications in which high temperatures and highly corrosive materials are present.
Membrane processes
By process, microfiltration membranes accounted for the largest share of the market last year, says Freedonia. The least selective of currently available membranes, microfiltration membranes are used widely as a pretreatment before finer separation processes. They also are popular as a low-cost alternative in applications requiring a relatively low level of purity.
"Because they perform the most basic types of membrane separation, microfiltration membranes are used in just about any market requiring separation," states Freedonia's Mapes.
However, the demand for reverse osmosis (RO) membranes will advance more rapidly as the highest level of purity increasingly is demanded in wastewater treatment and other applications. In fact, says the Freedonia report, the water and wastewater treatment market accounted for 55 percent of membrane demand in 2001. And the wastewater treatment segment is seeing rising industrial interest in reclaiming process components and recycling water.
"In terms of magnitude, membranes are contributing to wastewater treatment more than any other application," says OIT's Ozokwelu. "In this application, membranes are cost competitive with competing technology, the most common of which is steam stripping ," injecting steam into the wastewater and condensing hydrocarbon from the vapor."
Philip M. Rolchigo, chief technology officer for Osmonics, Minnetonka, Minn., says his customers want their RO processes to achieve two goals: lower capital costs and lower operating costs. Much of the company's research dollars are being spent on membranes that could operate at lower pressures than conventional RO membrane technologies, he adds.
"These systems become more cost-effective because you don't need to operate the systems at very high pressures," says Rolchigo. RO membrane systems also lower operating costs, he adds, because the pressure is directly related to the energy that must be applied to the process to purify the water.
Healthcare base
Some segments of the healthcare processing industry also rely heavily on membranes, especially the biotechnology sector, which regards the membrane as the separation tool of choice.
"It took decades for this technology to achieve bioprocess industry acceptance and to make significant inroads at scale," recalls WPI's Matson, "but there is no longer any argument about the potential of membranes in that arena ," they are being fully exploited now." The pharmaceutical industry also is increasingly hospitable to membrane technology, he adds.
According to The Freedonia Group, membranes are expanding from well-established niches into a variety of new uses. In the pharmaceutical market, process cost usually is not a primary factor, and manufacturers will spend whatever they need to accomplish the process effectively, adds OIT's Ozokwelu.
Some of the new uses for membranes include purification in the production of pharmaceuticals derived from human or animal sources. "Medical applications such as drug detection, leukoreduction and biotech applications also are growth areas for membrane separations technologies," says Freedonia's Mapes.
In fact, states Ozokwelu, just about all pharmaceutical syntheses involve biotechnology that use membranes. "It might be mentioned that special membranes are making the synthesis of some pharmaceuticals commercially viable," he adds. "This includes the separation of enantiomers or isomers of some pharmaceutical products that could not be effectively accomplished by any other means. This type of technology is outside the scope of industrial chemical or petroleum processing, for which low cost is the paramount consideration."
Up and coming
Membranes began to get serious attention from chemical engineers as process tools in the 1960s and 1970s, but their applications were almost entirely limited to aqueous process streams, says WPI's Matson. "For instance, a lot of attention was devoted to desalination of sea and brackish water with reverse osmosis membranes, [which were] initially based on cellulose acetate membranes [and] later pressed into service in acid gas separations," he explains.
The membranes of yesteryear, says Matson, generally were unsuited for use with organic solvents. Much of the recent R&D work ," on the part of both academia and membrane suppliers/developers ," has focused on the manufacture of membranes with higher temperature capabilities and greater resistance to the effects of organic solvents. These efforts and others are expanding membrane applications in the chemical industries.
The market for membranes in aqueous applications has been developed sufficiently, says Osmonics' Rolchigo. New breakthroughs in this area are unlikely. The next frontier for membrane technology is in the solvent area.
"We'll go replacing and augmenting distillation and liquid extraction," says Rolchigo. "I think the pharmaceutical industry will be the first industry to adopt it in a significant way. The value there will be quite significant. I think as the technology becomes more accepted and more cost-effective, you'll see it move more toward the food and petrochemical industries in a greater way," he adds.
The not-too-distant future also could see the promise of process intensification realized, driven by the need for on-demand, on-site chemical production, especially that of hazardous substances, predicts WPI's Matson.
Because they are able to downsize unit operations and process equipment, membranes are suitable platforms for process intensification, says Matson. Examples include fluid-phase contacting operations such as solvent extraction, the shrinking of certain processing operation dimensions (compare gas permeation with gas scrubbing, for instance), and the integration of unit operations where beneficial.