Plants Benefit From Better Cooling Tower Treatment
Our earlier article “Improve Your Cooling Tower Treatment,” http://bit.ly/2Z3k0Et, stressed the critical role that chemical treatment of cooling water systems plays for minimizing microbiological fouling, scaling and corrosion, and discussed a polymer chemistry that is replacing phosphate/phosphonate treatment. In this article, we look at two real-world examples of the benefits of that chemistry and point out the importance of not viewing corrosion inhibition in isolation.
But first, here’s a bit of background on the polymer chemistry. The original goal behind its development was to provide a more environmentally sustainable alternative to phosphates in cooling systems. The available formulations have flash points >200°F, aren’t U.S. Dept. of Transportation regulated and don’t contain California Proposition 65 materials. These products, typically applied at a dosage of 100 mg/L, have an LC50 (lethal concentration) on the common U.S. Environmental Protection Agency aquatic marker organisms in the range of 1,000–10,000 mg/L. In many cases, the chemicals can be formulated at mild pH values, making them less hazardous to handle than products with extreme pH values.
First Example
A large Southeastern industrial complex was using a polyphosphate corrosion inhibitor program. Influent water to the plant enters a large open pond and then goes to a settling clarifier. Chlorination takes place in the clarifier, with its discharge, approximately 4,000 gal/min, dosed with the corrosion inhibitor. After servicing a number of once-through cooling heat exchangers, a recirculating cooling tower and other process cooling needs, a portion of the water recycles back to the pond.
The phosphorus in the return water provided ample nutrients to support robust algae and microbiological growth, which in turn increased the biocide chemical treatment costs and fouled intake screens. Figure 1 shows the condition of the pond during the summer of 2014 when the phosphorus-based corrosion inhibitor program was in use. Similar to natural water systems with phosphate loading, the water has a deep, opaque green color.
Figure 1. Phosphorus in return water fostered substantial algae and microbial growth.
The total phosphate concentration in the pond was 3–4 mg/L. Despite the presence of the polyphosphate corrosion inhibitor, mild steel corrosion rates in the hot return of the once-through cooling water averaged a barely acceptable 8–10 mils/yr (mpy).To reduce biological growth, the facility converted the corrosion inhibitor program to 0.5–1.0 mg/L zinc in mid-September 2014. Corrosion rates on the zinc program dropped to an average of 2–4 mpy and the condition of the pond water improved considerably. Efforts to further reduce corrosion using higher levels of zinc alone led to some fouling of high temperature heat exchange surfaces.
At the end of May 2017, the site switched to a ChemTreat FlexPro non-phosphorus and non-zinc corrosion inhibitor program, which has the further advantage of being non-fouling. The inhibitor was applied at a concentration of 5 mg/L as formulated product. The initial corrosion coupon result was 3.4 mpy, comparable to that of the zinc corrosion inhibitor. Corrosion rate meter results in the clearwell supply water are in the 0.1–0.3 mpy range, the best ever achieved on any program in this application.
The appearance of the pond greatly improved after the conversion to non-phosphorus programs, as expected based on experience with phosphorus reduction in natural water bodies. Figure 2 shows the pond in the summer of 2017 after the switch. The total phosphate concentration is approximately 0.1 mg/L as PO4. With only the minimal natural phosphate loading in the source water, the pond water is crystal clear.
Figure 2. Switch to non-phosphate treatment program led to dramatic improvement in water clarity.
The elimination of zinc from the program deserves mention. Increasingly, discharge of some transition and heavy metals, including zinc, is coming under tightened scrutiny and regulation. However, removing zinc can have a downside because it protects galvanized cooling tower materials from corrosion. This is one of many aspects to consider in the design and chemical treatment of new cooling towers.
Second Example
A Gulf Coast natural gas liquids (NGL) fractionation plant separates raw or “Y-grade” NGL into its major components, principally ethane, propane, butane and higher condensates. Separation process economics require efficient cooling. Fractionation plants often use wet surface air coolers (WSACs) in which the process fluid in a closed-loop bundle is cooled by spraying open-loop water over the outside of the tubes while drawing air across the bundle to provide evaporative cooling. The heat exchanger coils are hot dip galvanized.
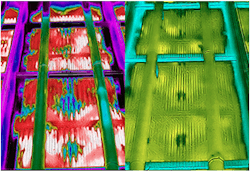
The plant relied on a phosphate-based treatment program for the open cooling water loop. This allowed considerable deposits of calcium phosphate and calcium carbonate to accumulate on the hot tube surfaces and water spray nozzles. The WSAC units were first cleaned for one month while the unit was in service by circulating a proprietary pH 6.5–7.5 cleaning chemistry containing the corrosion inhibitor to avoid damaging the galvanized tubes. Figure 3 shows thermal images of the water sprays and the tube bundle before and after cleaning. They indicate removal of most deposits and significantly improved water flow and spray coverage after the cleaning.
Similar cleaning took place on a depropanizer cooled by a conventional evaporative cooling tower. The system was so heavily scaled that both water flow and heat transfer were severely restricted, impacting production. Because this system didn’t contain galvanized surfaces, the cleaning process was more aggressive, briefly reaching a pH of 4.0. A FlexPro formulation was employed during and after the cleaning process to control corrosion. The cooling water flow was slowly restored over a period of ten days. Depropanizer approach temperatures decreased sharply during the initial ten days of cleaning and held steady for four weeks on the non-phosphorus treatment program. The previous phosphorus-based program then was reinstituted. Approach temperatures rose rapidly on the phosphorus program until it was replaced permanently by FlexPro after one week. The system has remained on the non-phosphorus program without fouling since late January 2017. Mild steel corrosion rates typically are <1 mpy, which is considered excellent by industry standards. The plant has credited the program for increased production of approximately 2,500 bbl/d by maintaining clean heat transfer surfaces.
Figure 3. Thermal images show state of WASC tube bundles and sprays before (left) and after (right) cleaning.
Other Important Chemistry
The passivating corrosion inhibitor chemistry described above has had many successful applications. However, no corrosion inhibitor treatment program typically can stand on its own without good control of other variables. First and foremost, proper microbiological control is paramount. Microbes, if allowed to grow and form colonies on cooling water surfaces, will render the corrosion inhibitor ineffective at the deposits. Furthermore, the microbes themselves can produce metabolic byproducts such as acids or hydrogen sulfide that will attack metals. Interestingly, evidence indicates that the efficacy of the non-phosphorus corrosion inhibitor increases with greater free available halogen concentrations, and a 0.5-mg/L residual as opposed to, say, a 0.2-mg/L residual can enhance microbial control and corrosion inhibitor performance. Operating at higher halogen residuals may require feed of a reducing agent such as sodium bisulfite to the cooling tower blowdown to comply with discharge permit regulations.
Application of the inhibitor to an existing system with rusted pipe sometimes poses difficulties because the rust will absorb the chemical. Achieving proper treatment efficiency may require cleaning older system pipes and other equipment.
Cooling water impurities can influence polymer performance. High iron content in the water may necessitate increased biocide residuals to defeat some microorganisms. Chloride isn’t problematic but high levels of sulfate may reduce effectiveness. (The reverse basically is true for phosphate/phosphonate programs.)
This points up the need for comprehensive water analyses to determine applicability of any treatment method. And, of course, impurities in the plant makeup will “cycle up” in cooling towers to concentrations several times greater. This impacts heat exchanger materials, too. For instance, some materials suitable for once-through cooling, such as 300-series stainless steels, may become unacceptable at the higher impurity levels. Fortunately now, with the development of advanced passivating chemistry, some flexibility in materials selection has returned. Even so, materials selection demands care during plant design. Spending a bit extra upfront on durable materials is far better than suffering process outages later due to corrosion of poorly selected metallurgy.
Look Beyond Phosphates
Phosphorus is an essential nutrient for algae growth and exhibits undesirable consequences in the aquatic environment. Fortunately, phosphorus isn’t absolutely essential to the control of corrosion or scale formation in cooling water systems. In fact, phosphorus has numerous limitations as a corrosion inhibitor. Moreover, its use in cooling systems presents many of the same issues as in the natural environment, including challenges regarding microbiological control, the potential for fouling heat transfer surfaces, inability to passivate lightly rusted surfaces, and little or no ability to control corrosion on alloys other than mild steel. As the two examples here illustrate, non phosphorus corrosion inhibitors offer important performance advantages, such as the ability to protect stainless steel, as well as environmental benefits. In addition, ongoing development of non-phosphorus cooling water treatment chemistries promises to increase their value.
BRAD BUECKER is a senior technical publicist for ChemTreat, Glen Allen, Va. RAYMOND M. POST, P.E., is the director of cooling technology for ChemTreat, Glen Allen, Va. Email them at [email protected] and [email protected].
This article is based on “The Development and Application of Non-Phosphorus Corrosion Inhibitors for Cooling Water Systems” presented by R. Post and R. Kalakodimi at the World Energy Congress, Atlanta, October 2017.