Take Better Care Of Your Low-Pressure Boiler
Many plants use low-pressure boilers to produce process steam for various applications, including heat for chemical reactors, evaporators, building spaces, etc. Often, sites pay less attention to the chemistry programs for these steam generators than to those for high-pressure units. Yet, contaminated condensate return, malfunctions of makeup water treatment systems, and other factors can cause many problems.
Plant staff largely understand that high-pressure boilers require high-purity makeup to minimize corrosion and scale formation in the harsh environment of the boiler and superheaters. In a way, this makes chemistry control more straightforward because the makeup water, boiler feedwater and steam all should meet stringent guidelines. The most-common makeup water scheme for modern high-pressure units uses membrane technologies (micro- or ultra-filtration for suspended solids removal and reverse osmosis for primary demineralization), followed by ion exchange or continuous electrodeionization polishing to produce high-purity water. (For pointers on proper treatment of high-pressure boilers, see “Don’t Get Steamed.”)
For lower-pressure steam boilers — here, we’ll focus on units up to about 600 psi that don’t drive turbines — makeup water treatment often is less rigorous. Usually, the leading concern is the potential for calcium carbonate (CaCO3) scaling (Figure 1), as illustrated by the following reaction of calcium ions (Ca2+) and bicarbonate alkalinity (HCO3-) that can occur in hot water systems and boilers:
Ca2+ + 2HCO3- + heat → CaCO3 ↓ + CO2 ↑ + H2O (1)
Figure 1. Precipitation from water can cause buildup on internal surface of heat exchanger tube.
So, for decades, a typical primary treatment method for industrial boiler makeup was sodium zeolite softening. In this process, the water passes through beds of ion exchange resin that swap the hardness ions calcium and magnesium for sodium. The softened stream, with the remaining impurities, including alkalinity, chloride ions (Cl-), sulfate ions (SO42-), silica (SiO2) and others, then feeds the boiler. Basic softening offers both advantages and drawbacks. The less-rigorous process, as compared to those techniques needed for high-pressure steam generators, saves the plant money in equipment and operating costs. However, many ions that aren’t removed by sodium softening can become problematic upon reaching the steam generator. Alkalinity may convert to carbon dioxide (CO2) in the boiler, which then carries over with steam. The CO2 can lower the pH in the condensate return, which leads to potential corrosion issues in these systems. With just sodium softening, the introduction of the remaining dissolved solids to the steam generator increases the general corrosion potential of the water due to higher conductivity, especially because the solids “cycle up” in drum boilers as steam is produced. Excess dissolved solids may induce foaming, which can boost impurity carryover to steam. Keeping the solids concentration at reasonable levels may require heavy blowdown. Chloride and, to a lesser extent, sulfate can be nasty impurities, especially in combination with oxygen in the boiler. The compounds also may concentrate under porous boiler-tube deposits, usually iron oxide corrosion products transported from elsewhere, e.g., condensate return systems, to induce acidic under-deposit corrosion (UDC). UDC continues to be a significant problem in industrial steam generators. (Low operating rates caused by the pandemic can spur UDC in other process equipment, see: “Keep Under-Deposit Corrosion Under Control.”)
Several methods can improve makeup water purity beyond basic sodium softening. Some of the older, established technologies are:
• Split-stream dealkalization. Such a setup places a sodium softener and strong acid cation exchanger in parallel, followed by a downstream forced-draft or vacuum decarbonator. Both sets of ion exchange resins remove hardness but the acid generated by the cation exchanger converts alkalinity to CO2, which is removed in the decarbonator. The process doesn’t remove chloride, sulfate or silica.
• Hot lime softening. This will remove most of the hardness, alkalinity, silica and iron. It doesn’t remove chloride.
• Ion exchange demineralization. Demineralizers come in various forms but, in general, if they have both cation and anion exchange capacity, they will remove most dissolved ions, including chloride and sulfate.
The first two methods use somewhat outdated technology. The development and maturation of membrane technologies, particularly for reverse osmosis (RO), have altered the landscape. Single-pass or especially two-pass RO can produce makeup water with very low dissolved solids, including the hardness ions and silica. Keys to successful operation of RO units are pretreatment to remove suspended solids ahead of the RO membranes and optimized chemical treatment to minimize scale formation on the membranes. Careful analysis of RO feedwater is critical for proper pretreatment equipment and chemical selection. Also, RO generates a near-steady wastewater stream that requires handling. For a plant with a cooling tower, the tower basin may serve as a good repository. Otherwise, a site may need alternative disposal methods.
A critical point to note, especially at an existing facility, is that making a change to higher-purity makeup for any application necessitates re-evaluation of chemical treatment programs. The change in water purity, even (seemingly) for the better, may lead to unforeseen consequences if not addressed properly.
Boiler-Water Treatment
Back in the 1930s, as power generating units increased in number and size, trisodium phosphate (Na3PO4 or TSP) became a popular boiler-water conditioning chemical for drum boilers. In the utility industry, phosphate treatment programs have undergone much evolution, with a return to just TSP, albeit in low dosages, common for modern units. For industrial boilers, phosphate treatment methods remain a strong choice.
A primary function of phosphate is to generate moderately alkaline conditions in the boiler to minimize general corrosion of carbon steel boiler tubes, drums, and headers:
Na3PO4 + H2O ↔ Na2HPO4 + NaOH (2)
Although TSP is the only recommended phosphate species for utility boilers (to minimize acid phosphate corrosion potential), in industrial units TSP may at times get blended with lesser amounts of disodium phosphate (Na2HPO4) and perhaps, although usually not recommended, even a bit of monosodium phosphate (NaH2PO4) to control excess formation of sodium hydroxide (NaOH), also known as caustic. Caustic can concentrate underneath porous boiler tube deposits and induce direct corrosion of the boiler metal, in this case due to excess basicity as compared to acidic chloride attack.
A second function of phosphate, which is particularly important for units in which hardness ions periodically may ingress, is to control scale formation. Phosphate, and the alkalinity produced by its reaction with water, can react with hardness ions to form soft sludges as opposed to hard scale. Often recommended with phosphate treatment are sludge conditioners consisting of water-soluble polymers that help keep solids in suspension by a combination of dispersion, crystal modification and sequestration. Such sludge conditioners can enable effective blowdown of otherwise troublesome iron particles from condensate return system corrosion. These polymers sometimes can serve as a stand-alone treatment, particularly if hardness ingress isn’t an issue. Another technique successfully employed at times in industrial drum units, but not utilized much today, is chelant chemistry, in which the chemicals directly bind with metals to keep them suspended. Ethylenediaminetetraacetic acid (EDTA) is the most widely known chelant, and has served for many applications often outside the steam generation industry. However, improper use or control of chelants can cause localized corrosion of boiler components.
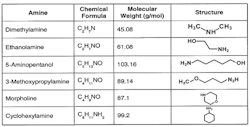
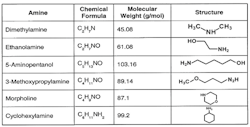
Figure 2. Boasting higher molecular weights than ammonia, these compounds won’t flash off as much.
The upshot is that several possibilities exist for boiler water treatment. The proper choice depends upon a variety of factors that include boiler design and pressure, makeup-water treatment sophistication and reliability, and the potential for impurity ingress from condensate return. These factors require careful evaluation for each case. A “one size fits all” approach to treatment selection can lead to problems.
Film-forming products (FFPs) for steam generator treatment are under development. These compounds provide a protective hydrophobic layer to metal surfaces to inhibit corrosion. Reports of successful applications of some FFPs continue to emerge but use requires diligent planning and monitoring.
Feedwater And Condensate-Return Treatment
A large influence on boiler water chemistry can come from ingress of corrosion products or impurities from the feedwater system and condensate return. A top priority is maintaining moderately alkaline conditions in these systems to prevent general corrosion of carbon steel, the typical material for feedwater and condensate return piping. In the power industry, the common pH-conditioner is ammonia, which raises the feedwater pH via the following reaction:
NH3 + H2O ↔ NH4+ + OH- (3)
Because this is a reversible reaction, the alkalinity increase is limited, which usually minimizes excessive corrosion to steel in the event of a chemical feed upset. (Copper alloy corrosion is a completely different story.) For industrial boilers, neutralizing amines (Figure 2) are a common choice for condensate/feedwater pH conditioning. These are small-chain organic molecules with an ammonia group attached to or embedded within the compound.
The amines all have a higher molecular weight than ammonia and, thus, won’t flash off nearly as extensively as ammonia does to steam — although each has its own distribution ratio, i.e., the amount that remains in the water versus that which departs with steam, whose properties are a function of temperature and pressure. The products also have different basicities, which provides flexibility in selecting a treatment program. Careful evaluation of boiler operating and design conditions is necessary to select the most appropriate amine or amine blend. Some compounds aren’t allowed if the steam can directly contact food and other consumable products.
Neutralizing amines often are very important for minimizing corrosion in condensate return systems, particularly if the boiler water contains significant alkalinity. Carryover of CO2 to steam can depress the pH in the recovered condensate; substantial iron corrosion may result unless the pH is adjusted with a neutralizing chemical.
The potential exists at many plants for impurity ingress from process heat exchangers or other sources. Some form of condensate polishing can prove beneficial but determining the constituents to remove requires careful analysis. If iron particulates from condensate return system corrosion are the major issue, then fabric filter techniques might suffice. Ion exchange can remove dissolved ions such as sodium, hardness, chloride, silica, etc. If organic compounds are the problem, activated carbon filtration or specialty exchange resins may be the answer. Factors that influence condensate polisher selection, such as flow rate, temperature and potential for media fouling, again are specific to each plant.
Don’t Forget Dissolved Oxygen
The issue of control of dissolved oxygen (DO) can be a bit thorny. For many years, the accepted wisdom in the power industry was to remove all DO from feedwater. Facilities relied on mechanical (deaerators) and chemical (oxygen scavengers/reducing agents) methods to accomplish the goal of zero oxygen at the inlet to the boiler economizer. However, in the 1980s (and continuing to this day), issues regarding flow-accelerated corrosion (FAC) began to appear; in some cases FAC-induced failures caused fatalities. This corrosion results from gradual dissolution of the protective oxide layer (magnetite, Fe3O4) that forms on carbon steel at startup but leaches away at flow disturbances, e.g., elbows, in the reducing conditions established by an oxygen scavenger. (Temperature and pH also are important factors.) In the meantime, researchers discovered that for units with high-purity makeup (less than 0.15 or 0.2 µS/cm cation conductivity depending upon the particular program employed), some dissolved oxygen in the condensate actually proves beneficial, causing carbon steel to form a tight, reddish-colored, protective oxide layer, different than the gray-black magnetite normally observed. Thus, these mildly oxidizing chemistry treatments now are recommended for almost all high-pressure utility steam generators unless the condensate/feedwater system contains copper alloys.
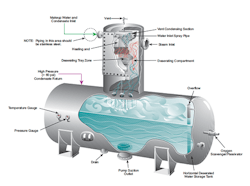
Figure 3. Steam scrubbing of makeup water and condensate return injected at the top of the tray section removes dissolved oxygen.
However, industrial boilers usually receive less than high-purity makeup water, so DO control is quite important to minimize oxygen attack of steam generator components. Most systems come with a mechanical deaerator (Figure 3), which, when operating properly, should reduce the DO concentration to 7 ppb.
In addition, normal practice is to use a chemical oxygen scavenger, often either un-catalyzed or catalyzed sodium sulfite (Na2SO3):
2Na2SO3 + O2 → 2Na2SO4 (4)
A common injection point is the deaerator storage tank. The combination of mechanical and chemical methods usually can protect the steam generator against significant oxygen corrosion.
Space limitations prevent a comprehensive discussion of chemistry monitoring here. However, one critical item is iron monitoring, which the power industry has adopted as a standard parameter and which other plants should use as well. On-line and grab sample methods are available to track corrosion products and determine the effectiveness of pH-conditioning and oxygen-control methods. Steam generator protection during outages also is important; “Plant Combats Corrosion in Idled Boilers,” provides some insights on this.
BRAD BUECKER is a senior technical publicist for ChemTreat, Glen Allen, Va. CHAD FRIERSON is a technical consultant with ChemTreat, Glen Allen, Va. Email them at [email protected] and [email protected].