Reaction & Synthesis: Quickly Estimate Reagent Addition Time
Batches often have a step in which a reagent chemical is added to a mixture being stirred and reacts immediately (with little accumulation) — with the rate of addition controlled by the ability to remove heat. A maximum temperature is specified with full cooling applied to the reactor’s jacket. If the reagent chemical is dilute enough to cause a significant level change, then the wetted area for heat transfer will not remain constant.
When the reaction rate is much faster than the feed rate, the added reagent is immediately converted to product by spontaneous reaction with the substrate previously charged to the reactor. A negligible buildup of the reagent occurs in the reactor during the addition. The heat production rate is directly proportional to the feed rate of the reagent as limited by the heat transfer of the jacket. The reagent feed rate can be raised as the volume increases and provides more wetted area for heat transfer.
Deriving The Equation
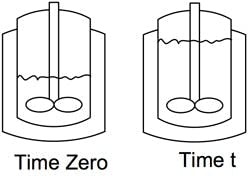
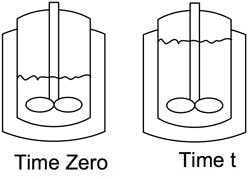
Figure 1. Equation assumes that batch always is on the straight wall of vessel.
For simplification, let’s assume the liquid mixture in the reactor prior to addition is on the straight wall as shown in Figure 1. For purposes of integration, let’s also assume the heat of reaction (ΔH), liquid density (ρ), overall heat transfer coefficient (U) and temperature differential (ΔT) between the utility (jacket) and process (tank) remain constant.
This situation is the reverse of a batch distillation — for which the equations and their derivation have been published previously [1]. The solution is:
At/A0 = e–t/Θ (1)
where Θ = ρ×D×ΔH/(4U×ΔT) (2)
The differences versus batch distillation are in the definition of the terms ρ, ΔH and ΔT. Let’s discuss each of these.
Liquid density. In batch distillation, ρ is the density of the liquid in the vessel. For reagent addition, this term is the net weight added during the addition versus the observed volume change of the reaction mix. For the special case where the reagent and reaction mixture mix ideally with a zero volume change, the density equals that of the reagent.
Enthalpy change. In batch distillation, ΔH is the heat of vaporization of the evaporated solvent. For reagent addition, it is the heat of reaction expressed as unit of heat versus the net weight added. The heat of reaction must be calculated at the temperature of reaction and must include all enthalpy effects such as the sensible heat from a reagent below reaction temperature, heat of dilution, evaporative cooling when there is a byproduct off-gas and, of course, the chemical heat of reaction.
Temperature difference. The ΔT term is the same and constant for both batch distillation and reagent addition. Typically in batch distillation, steam is the heating medium and calculation of this term is straightforward because both the process and jacket are isothermal. In reagent addition, a liquid commonly is the coolant and the jacket supply and outlet temperature are unequal. In this case, the difference is determined by a log mean temperature calculation.
For a simple case of water flowing once through a jacket, the following relationships apply:
TO = TP + (TS - TP)/K (3)
where K = exp [(UA)/(WCp)] (4)
Eq. 4 includes the wetted area (A). Under a rigorous analysis, the temperature difference is not constant. However, as an approximation, the log mean temperature difference can be calculated at both the lowest level (A0) and highest level (At) in the integration. For practical problems, the observed disparity in temperature difference will be slight and the lower value can be used to provide a conservative estimate of addition time. An example will illustrate this.
Many reactors have heat/cool modules that enable the jacket inlet temperature to differ from the coolant supply temperature. In such cases, substitute equations that are available in Reference 2 for Eq. 3 and Eq. 4.
An Example
Molten sodium metal is added to water to make a sodium hydroxide solution — with the temperature controlled isothermally at 77°F during the addition. The reaction chemistry is:
Na (liq.) + H2O (liq.) → NaOH (aq.) + ½H2 (gas)
The reaction occurs in a vessel with a 5-ft outer diameter and a ¼-in.-thick shell, equipped with a bottom ASME F&D head. The straight wall holds 144.5 gal/ft of liquid height. The bottom head holds 73.9 gal. The straight side has a wetted area of 15.7 ft2/ft of liquid height. The outside surface area of the bottom head is 23.2 ft2. Table 1 presents a heat and material balance for this reaction.
Let’s now estimate the addition time, assuming an overall heat transfer coefficient of 80 BTU/hr/ft2/°F and 25,000 lb/hr of 41°F chilled water flow (Cp = 1) to the jacket.
Step 1. Calculate the liquid density, ρ.
Per Table 1, the change in volume is 712.0 - 795.9 = -83.9 gal = -11.2 ft3. The change in weight is 2,928.9 + 5,276.9 - 6,610.3 = 1,595.5 lb. The liquid density for this reaction is 1,595.5 lb/(-11.2 ft3) = -142.5 lb/ft3. (Surprise! The density is a negative number because the total volume shrinks after the addition.)
Step 2. Calculate the heat of reaction.
Per Table 1, the heat liberated is the sum of the product enthalpies minus the sum of the reactant enthalpies, i.e., [0.0 + (-82.0) + (-14,540.7) + (-36,050.0)] - [179.0 + (-45,159.4)] = -5,692.3 kBTU. The net weight added to the reactor is 1,595.5 lb. (Surprise! It doesn’t equal the weight of the sodium metal because of the evolution of hydrogen gas.) The heat of reaction is then -5,692.3 kBTU/1,595.5 lb = -3.568 kBTU/lb or -3,568 BTU/lb.
Table 1. Sodium added to vessel causes an immediate reaction.
Step 3. Calculate the height on the straight wall.
Start of reaction: (795.9 - 73.9)/144.5 = 5.00 ft
End of reaction: (712.0 - 73.9)/144.5 = 4.42 ft
Step 4. Calculate wetted areas.
Start of reaction: 5.00×15.7 + 23.2 = 101.7 ft2
End of reaction: 4.42×15.7 + 23.2 = 92.6 ft2
Step 5. Calculate K factors. (See Eq. 4.)
Start of reaction: K = exp (80×101.7)/(25,000×1) = 1.385
End of reaction: K = exp (80×92.6)/(25,000×1) = 1.345
Step 6. Calculate jacket outlet temperatures. (See Eq 3.)
Start of reaction: TO = 77 + (41 - 77)/1.385 = 51.0°F
End of reaction: TO = 77 + (41 - 77)/1.345 = 50.2 °F
Step 7. Calculate ΔT.
Start of reaction: [(77 - 41) - (77 - 51.0)]/ln [(77 - 41)/(77 - 51.0)] = 30.7°F
End of reaction: [(77 - 41) - (77 - 50.2)]/ln [(77 - 41)/(77 - 50.2)] = 31.2°F
The two values differ by less than 2%. Per the integration assumptions, the temperature will be treated as constant — using the lower value, 30.7°F, in subsequent calculations to give a conservative estimate of the addition time.
Step 8. Calculate Φ. (See Eq 2.)
First, calculate the tank’s inside diameter: 5 - 2×¼×⅟12 = 4.9583 ft
Φ = [(-142.5)×4.9583×(-3,568)]/(4×80×30.7) = 256.6 hr
Step 9. Rearrange Eq. 1 to solve for time.
t = -Φln(At/A0)
Step 10. Plug in values and calculate addition time.
-256.6×ln(92.6/101.7) = 24.1 hr
MIKE GENTILCORE is a principal engineer with Mallinckrodt Pharmaceuticals, Hazelwood, Mo. LUIGI GRIPPA is a consultant and trainer based in Milan, Italy. E-mail them at [email protected] and [email protected].
NOMENCLATURE
A area, ft2, m2
Cp Coolant heat capacity, BTU/(lb×°F), J/(Kg×-°C)
D Tank inside diameter, ft, m
ΔH Heat of reaction, BTU/lb, J/kg
K Flow rate correction per Eq. 4, dimensionless
t Temperature, °F, °C
ΔT Temperature difference (jacket - process), °F, °C
T Time, hr
U Overall heat transfer coefficient, BTU/(hr×ft2×°F), W/(m2×-°C)
W Coolant flow rate, lb/hr, kg/s
ρ Liquid density, lb/ft3, kg/m3
Θ Time constant per Eq. 2, hr
Subscripts
0 Time zero
f Formation
t Time t
S Coolant supply (jacket inlet temperature)
O Coolant return temperature (jacket outlet temperature)
P Process temperature (reaction temperature)
REFERENCES
1. Gentilcore, M. J., “Quickly Estimate Batch Distillation Time,” Chemical Processing (April, 2013)
2. Gentilcore, M. J., “Estimating Heating and Cooling Times for Batch Reactors,” Chemical Engineering Progress (March 2000).