Catalyst Provides Simple Route to Amines
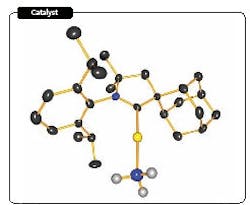
Figure 1. Carbene-based catalyst contains gold (yellow), nitrogen (blue), hydrogen (gray) and carbon (black). Source: University of California, Riverside.
A novel catalyst may revolutionize the way amines are commercially made, hope researchers at the University of California, Riverside, Calif. Their laboratory trials indicate that the catalyst — a gold atom linked to a cyclic alkyl amino carbene (Figure 1) — provides high yields and good selectivity for the reaction of ammonia and allenes under mild conditions. Moreover, adjusting the ammonia/allene ratio allows significant control over selectivity, notes Guy Bertrand, distinguished professor of chemistry at the school, who heads the team of researchers. The catalyst also is suitable for reacting alkynes and can be used to produce most amines.
Current commercial processes rely on hydrochloric acid and generate three times as much waste as product. “Our ‘green chemistry’ method, however, produces no waste, which makes it inexpensive. Moreover, the reaction is a quick, one-step reaction, and you need a tiny amount of catalyst to do the trick,” he says.
The researchers have used the catalyst to react ammonia with 1,2 propadiene to form mono, di- and triallylamine. The gram-scale tests took place at 110° to 180°C and atmospheric pressure, usually ran for 10 to 16 hours, and gave amine yields of around 80% to 90%, says Bertrand, who stresses that yields haven’t been optimized. Fine-tuning of reactant ratio provided selectivities for allylamine and triallylamine of 86% and 91%, respectively, with further improvements possible, he adds. Unreacted ammonia can be recycled.
The catalyst is thermally stable and used at low concentrations (1 mol%), he notes. At the small scale of the trials, it hasn’t been worth trying to recover the material. However, in a commercial process — where the catalyst likely would be put on a support — its recovery certainly is possible, he says.