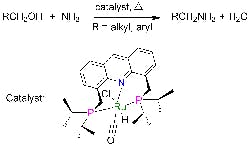
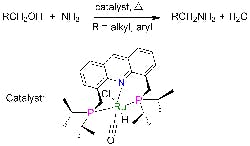
Catalyst Simplifies Amines Production
Primary amines play a crucial role in making pharmaceuticals, fine chemicals, polymers, emulsifiers and other materials. However, terminal primary amines, which are the most useful, boast high reactivity, making their selective synthesis challenging because reaction can continue to secondary and tertiary amines, note researchers at the Weizmann Institute of Science, Rehovot, Israel. Fortunately, a new catalyst promises significant improvement, says David Milstein, a professor in the Department of Organic Chemistry there — it enables direct production at high yields under mild conditions, obviating the multiple steps and toxic reagents now required.He and co-worker Chidambaram Gunanathan have discovered a ruthenium-based catalyst that facilitates the reaction of ammonia with alcohols to form primary amines and water without byproducts. Small-scale laboratory tests have provided amine yields reaching up to 96% at 135°C and 7.5 atm. “The reported yields are not optimized, and it is likely that they can be improved by variation of reaction conditions such as temperature and reaction time,” notes Milstein, adding that no catalyst poisoning has been observed. “In commercial processes, much higher temperatures and pressures are employed, and the yields are lower, due to large amounts of secondary and tertiary amines as well as alkene (by alcohol dehydration) and alkane side products,” he says.