Nanocatalyst Gains Greater Reactivity
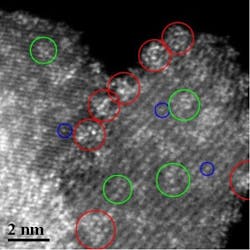
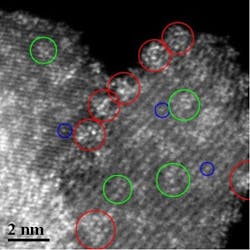
Providing the right surface coverage of sub-nanometer clusters of tungsten oxide on a zirconium oxide support (Figure 1) makes the catalyst five times more reactive for n-pentane isomerization, reports an international group of researchers. And the strategy promises to bolster a variety of other acidic reactions, says Michael Wong, a professor at Rice University, Houston, a part of the team.
Refineries certainly stand to gain from more efficient production of isopentane, which is used in gasoline. "We have a way to make a better catalyst that will improve the fuels they make right now," says Wong. "At the same time, a lot of existing chemical processes are wasteful in terms of solvents, precursors and energy. Improving a catalyst can also make the chemical process more environmentally friendly."
The key is achieving the optimum surface coverage of the nanocatalyst on the support, notes Wong, whose team at Rice collaborated with researchers at Lehigh University, Greece's Centre for Research and Technology Hellas, and the DCG Partnership of Texas. Details appear in a recent paper in the Journal of the American Chemical Society.
The greater reactivity for n-pentane isomerization "translates into a higher concentration of isopentane and, at the same time, a lower concentration of byproducts. The benefits from this double effect (higher turnover rates and higher selectivity) are great and we believe we can significantly reduce industrial separation-unit costs if we can further improve our material synthesis techniques," Wong notes.
He says now that the catalyst formula is "just right."
Recent studies show that after two catalytic cycles, overall activity remained practically the same, adds Wong, a professor of chemical and biomolecular engineering and of chemistry. "We expect the catalyst to have a longer life due to the absence of chloride species (used in industrial isomerization catalysts) that eventually leach out causing serious regeneration issues," he says.
Besides pentane isomerization, other chemical reactions that might benefit from the catalyst include acidic reactions such a paraffin, olefin and aromatic compound isomerization, dehydration of paraffins to olefins, esterification reactions, hydrolysis and metathesis.
"These are reactions of great importance for the petrochemical industry, especially in fuel enrichment technologies. We are starting to investigate metathesis in our lab, because we think that other forms of the surface tungsten can be good for this reaction," says Wong.
Wong and his team are also investigating optimization of the surface coverage of other catalytic nanomaterials that are used on supports. "We've started putting molybdenum oxide and vanadium oxide in place of tungsten oxide using a new synthesis technique we are developing," he says.
Producing the catalyst on a large scale should be straightforward, notes Wong. Lab samples were made using conventional dry impregnation — the most common method used commercially. Industrial firms already have expressed interest in cooperating on further development.
Using the catalyst in existing reactors doesn't require any major modifications, he adds.