Plasmonic Sensors Double as Catalyst
A nanoscale octopod consisting of plasmonic gold particles with palladium tips could lead to more efficient industrial processes, believes a researcher from Rice University, Houston.
“Plasmonic particles are magnets for light,” says Emilie Ringe, assistant professor of materials science and nanoengineering and chemistry at the university. “They couple with light and create big electric fields that can drive chemical processes. By combining these electric fields with a catalytic surface, we could further push chemical reactions. That’s why we’re studying how palladium and gold can be incorporated together.
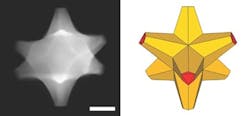
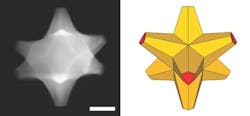
Figure 1. This octopod, which has a gold core and a gold/palladium alloy surface, has both plasmonic and catalytic abilities. Source: Ringe Group/Rice University.
“If you simply mix gold and palladium, you may end up with a bad plasmonic material and a pretty bad catalyst, because palladium does not attract light like gold does,” she explains. “But our particles have gold cores with palladium at the tips, so they retain their plasmonic properties and the surfaces are catalytic.”
Ringe, along with researchers from the U.S., U.K. and Germany, made 3D maps of the electric fields produced by exciting the plasmons and found that strong fields were produced at the palladium-rich tips, where plasmons were the least likely to be excited. A recent Scientific Reports article provides more details.
The octopods also are well-suited for extended use as catalysts. “These structures are really robust, as the core is mainly gold. We have seen no structural changes over about a year,” notes Ringe. Susceptibility to poisoning is not known she admits, but says the presence of gold (i.e., the fact they are not only Pd) may have a beneficial effect.
Researchers still must test the catalytic performance (e.g., yield) of a particular multifunctional plasmonic-catalytic system for a specific reaction. However, she says, “we are very excited to do so soon.” Other challenges include figuring out how these octopods actually perform catalytically, and how their performance depends on the light energy used, she adds.
While using the sun to excite and enhance chemical reactions is ideal, lasers or other light sources also can work, Ringe notes. “The idea is that when plasmons are excited, they create strong electric fields that can influence the behavior of molecules. Moreover, plasmons decay into heat or hot electrons, that can further enhance chemical reactions.”
Further research will produce multifunctional nanoparticles in a variety of shapes that can be greatly refined for applications. Ringe’s lab currently is working on a metal catalyst to turn inert petroleum derivatives into backbone molecules for novel drugs.
“We have two years of funding from the ACS-Petroleum Research Fund to look into how we can turn naphthalene into a more reactive chemical feedstock, which can then be used to create more complex molecules,” she says.
Ringe believes making the catalyst on an industrial scale doesn’t pose any major issue, but admits using gold is expensive. “There are some exciting new plasmonic materials now based on earth-abundant elements [and compounds], such as aluminum and titanium nitride. Maybe the next step should be to look at how to use less gold and cheaper metals,” she muses.
“Photocatalysis will, I believe, open new doors to greener processes,” she adds.