Method Makes Carbon Dioxide a Star
A one-step process produces three- and six-arm star-shaped carbonate compounds from carbon dioxide at good yield and with high selectivity at mild conditions, report researchers at the Agency for Science, Technology and Research (A*STAR), Singapore. It may enable using the greenhouse gas to make molecules applicable in catalysis, coatings and drug delivery, they believe.
“The carbon-carbon triple bonds are chemically reactive; hence, currently we are planning to use the star-shaped molecules as starting materials to synthesize more functional star-shaped materials,” says He-Kuan Luo, leader of the team at A*STAR’s Institute of Materials Research and Engineering. Work should begin in early summer and take about a year, he notes.
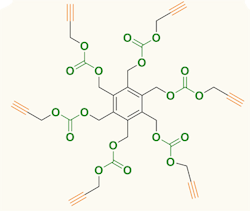
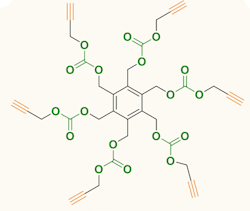
Figure 1. Carbon dioxide serves as feedstock for producing six-arm star-shaped compounds. Source: Agency for Science, Technology and Research, Singapore.
The compounds produced by the team contain a benzene ring with either three or six identical arms, each terminated with a 1-alkynyl group. Synthesis involved reacting tri- or hexa-bromomethyl benzene and propargyl alcohol at room temperature under CO2 at atmospheric pressure with tetrabutylammonium bromide as a promoter. The synthesis took two days for the three-arm compound and four days for the six-arm one; yields were 71% and 65%, respectively. More details appear in an article in Chemical Communications.
“The current key challenge is to shorten the reaction time by searching for a more-efficient promoter and/or base that can accelerate the reaction rate because the reaction rate is still slow for practical applications,” explains Luo. “Ultimately, quantitative yield (relative to the halide substrate) should be attained because the reaction has high selectivity and the reaction is mild.”
[javascriptSnippet ]
Making the star-shaped compounds on a larger scale poses some issues, he admits. The process uses dimethylacetamide (DMA) as solvent and, because of its high boiling point (165°C), recycling would cost more than for a lower-boiling solvent such as tetrahydrofuran. In addition, the six-arm carbonate’s starting material (hexa-bromomethyl benzene) has poor solubility in DMA, which may further slow down the reaction; the solvent volume can’t be reduced significantly, Luo adds.
For details on other emerging technology using CO2 as a feedstock, see: “Better Bioprocess for Acetic Acid Beckons,” “Better Catalyst for Carbon Dioxide Reduction Beckons,” and “Carbon Dioxide Gets a New Fizz.”