Better Propene Process Beckons
A decline in the availability of coproduct propene from naphtha cracking opens up a market opportunity for an alternative approach for supplying the alkene, say researchers at the University of Wisconsin — Madison (UW-Madison). They have developed a process for the oxidative dehydrogenation of propane (ODHP) that markedly outperforms others. The key is use of boron nitride as catalyst; it provides unique and unexpected catalytic properties, they note.
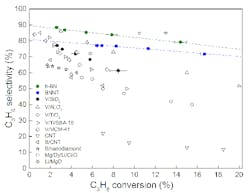
The team at Madison, led by chemistry and chemical engineering professor Ive Hermans, used hexagonal boron nitride (hBN) and boron nitride nanotubes as catalysts. The boron-nitride-catalyzed process provides higher selectivity than that achieved with the supported vanadium oxide catalysts typically used for ODHP, they report (Figure 1). Moreover, their process yields ethylene as coproduct instead of carbon dioxide and other undesirable byproducts formed with other catalysts. The boron-nitride-catalyzed process gave 79%-propene and 12%-ethene selectivity at 14% propane conversion. More details on the process appear in a recent article in Science.
[callToAction ]
“Boron nitride catalysts are nontoxic, they don’t contain precious metals, and they reduce the temperature of the reaction, resulting in energy savings,” notes graduate student Joseph Grant, a member of the team that developed the process.
Hermans points out another plus: “What is great about this result is that we see catalytic activity using commercially available hBN. This material is already produced in large-scale quantities. Scale-up of the catalyst is a typical hurdle for many catalytic reactions. So this is at least one problem that people seemed to have cracked.”
Figure 1. Boron nitride catalysts provide better results for oxidative dehydrogenation of propane than vanadium oxide catalyst. (Open shapes indicate data from other works.) Source: University of Wisconsin — Madison.
“We will be collaborating with a process engineering group here at UW-Madison to perform a techno-economic analysis to see how close we are to make this feasible for industrial implementation,” states Hermans. “In parallel, we are trying to further increase the selectivity and activity to maximize the space-time yield… Though we see high selectivity to olefins propene and ethene, we’d still like to higher selectivity to only propene.”
“We foresee interested parties running large laboratory-scale experiments within the next 2–4 years… If everything goes as we hope and larger laboratory-scale trials are successful, pilot-scale plants might be built within 5–10 years,” says Hermans. A company, not the university, would build and run such plants, he adds.