Copper Catalyst Steps Up Yields
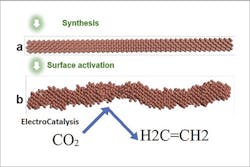
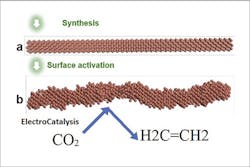
Figure 1. System synthesizes the smooth nanowire and then activates it by applying a voltage to get the rough stepped surface that is highly selective for CO2 reduction to ethylene. Source: Huang and Goddard.
Nanoscale copper wires with specially shaped surfaces can catalyze a chemical reaction to convert carbon dioxide into ethylene, says a research team from the California Institute of Technology (Caltech), Pasadena, Calif., and the UCLA Henry Samueli School of Engineering and Applied Science, Los Angeles.Using copper to kickstart the CO2 to ethylene conversion traditionally has posed problems. The initial chemical reaction produces undesirable hydrogen and methane byproduct, and the ethylene conversion efficiency wanes as the system continues to run.
To address these issues, the researchers created a “step” pattern design of the copper nanowires — similar to a set of stairs arranged at atomic scale. This step pattern across the nanowires’ surfaces remains stable under the reaction conditions. An article detailing the system’s durability and selectivity in producing ethylene appears in Nature Catalysis.
Computational studies of the reaction show the shaped catalyst favors the production of ethylene over hydrogen or methane. The researchers also report a CO2-to-ethylene conversion rate of greater than 70%, much more efficient than previous designs that yielded at least 10% less under the same conditions. Electron microscopy and OH- adsorption cyclic voltammetry confirmed the stepped Cu nanowire surface remained stable for 200 hours during the electrochemical CO2 reduction reaction.
“The idea of using copper to catalyze this reaction has been around for a long time, but the key is to accelerate the rate so it is fast enough for industrial production,” says William A. Goddard III, Caltech professor of chemistry, materials science, and applied physics. “This study shows a solid path towards that mark, with the potential to transform ethylene production into a greener industry using CO2 that would otherwise end up in the atmosphere.”
“…It is estimated that ~65% full cell efficiency of ethylene production from CO2 with 500 mA/cm2 is required for this process to be cost-competitive,” adds Yu Huang, professor of materials science and engineering at UCLA. “This translates to about 80% efficiency in cathode half-cell reaction. Our demonstrated Faraday Efficiency for C2H4 is close to the target, at ~77% at pH=6.8.”
Huang cites three key challenges to further advance the process: 1) scaling up production of the stepped Cu surface; 2) engineering a full-cell configuration and condition that work for the Cu stepped surface (likely at neutral conditions); and 3) reducing the reaction’s energy consumption or using solar energy to drive the reaction. “To incorporate the material design in a conventional electrolyzer likely takes about five years; using solar energy to drive the CO2 to fuel transformation likely needs longer time,” she suggests.
The economics of scaling up also poses some issues. “The current approach uses colloidal synthesis which can be readily scaled up, but the process is likely more costly than one would like. Ideally, we should design a cheaper process to achieve the same topography to be more cost effective,” Huang muses.
Furthermore, metal ion impurities are likely to pose a problem in this system, so, pure Cu is preferred. “Similar to the existing water electrolyzers, pure water is usually used,” notes Huang.
Using stepped nanowires for other copper-catalyzed reactions holds promise, believe the researchers.
“Although stepped (high-index) facets of heterogenous catalysts have been recognized to exhibit high catalytic activity, they are generally believed to be unstable. Our studies showed and explained, for the first time, their long-term stability with exceptional performance. This study opens up new possibilities and great potential for the generation and application of unique nanoscale surfaces that are both highly active and highly stable, which will be attractive to the broad community of material scientists and chemical engineering working on catalysis,” believes Huang.
Next, the team will focus on better understanding and designing the optimum microenvironment to facilitate CO2 reduction to ethylene with high efficiency, in a practical full cell setting. “We hope to achieve the goals in 2–3 years at the lab scale,” says Huang.
“We are interested in collaborating with industries on this project to scale up production of the catalysts and put them to actual use, but we have not been approached yet. One reason may be the demonstrated efficiency is still in the lab and at half-cell level,” Huang concludes.