Better Acrylates Synthesis Beckons
A highly chemoselective transesterification reaction with high yields and mild reaction conditions promises to improve the manufacture of industrial acrylates, say its developers at Nagoya University, Nagoya, Japan.
The new reaction minimizes undesired Michael reactions that occur in industrial production and also does away with toxic metal salts currently used in (meth)acrylate — i.e., acrylate or methacrylate — ester synthesis.
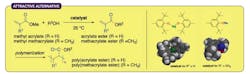
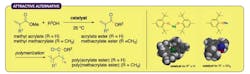
Figure 1. New route avoids use of toxic metal salts and minimizes undesirable Michael reactions. Source: Nagoya University.
Michael additions can foster the synthesis of C-C bonds but also cause unwanted addition and polymerization reactions.
“Millions of tons of (meth)acrylate esters are produced annually and are among the most important manufactured chemicals around,” notes Kazuaki Ishihara, a professor in the laboratory of catalysis in organic synthesis at Nagoya University. “Their transesterification, using alcohol and a catalyst, fine-tunes their properties, producing a wide range of (meth)acrylate esters.” Such properties include flexibility, transparency and weatherability.
Ishihara and his colleagues found that sterically bulky sodium and magnesium aryloxides worked very well at 25°C as nontoxic alternatives — producing a broad range of (meth)acrylate esters depending on the type of alcohol used in the reaction (Figure 1). The researchers employed numerous alcohols, including a wide selection of functionalized primary and secondary alcohols, diols, triol and tetraol identified by extensive substrate screening. They relied on computational density functional theory calculations to maximize chemoselectivity and minimize unwanted Michael additions.
The Nagoya team observed an almost 97% preference for transesterification over Michael additions.
The initial work took place without industrial collaboration, says Ishihara. However, a number of chemical companies have contacted the team since a paper on the development appeared in a recent issue of ACS Catalysis.
“So now we have contacted several chemical companies looking at the possibility of collaboration. The next stage of the work is moving to a pilot scale with them,” he adds.
Ishihara foresees two issues dominating a move to pilot scale and beyond.
The first is that his team used molecular sieves to remove the methanol that is generated alongside the (meth)acrylate esters. “Their use at a large scale should be avoided and another method is needed to remove the methanol,” he stresses.
Finding ways to recover and reuse the catalysts is important, too, notes Ishihara.
Meanwhile, his team also continues to study other catalysts to try to find any that might be more effective in the transesterification of (meth)acrylates.