Greener Propylene Production Looms
Oxidative dehydrogenation has long been considered a more-energy-efficient way to make propylene from propane, but the method produces small yields. Now, a new approach from researchers at Northwestern University, Evanston, Ill., results in higher yields while also using less energy. The findings could support more-energy-efficient production processes for many plastics, and could benefit smaller chemical plants where energy consumption is very important and current engineering strategies may not be feasible, say the researchers.
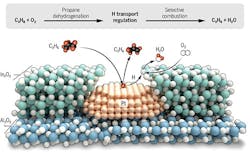
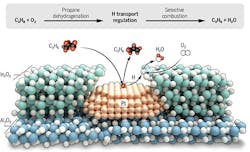
“Instead of searching for the right catalyst, we deconstructed the oxidative dehydrogenation reaction down into two components — dehydrogenation and selective hydrogen combustion — and then designed a tandem material that does both reactions, in a particular order. This produced the highest yields of propylene ever reported,” says Justin Notestein, professor of chemical and biological engineering at Northwestern and co-corresponding author on the research.
Tests produced 30% yield from a single pass through the reactor at 450°C, compared to 800°C for traditional propylene production; more than 75% of the carbon atoms in the propane converted to propylene. By comparison, heating propane in the absence of oxygen doesn’t produce yields greater than 24%, and the catalysts often are unstable, the researchers note.
The approach uses a platinum-based catalyst that selectively removes hydrogen from propane to make propylene, and an indium oxide-based catalyst that selectively burns the hydrogen, but not the propane or propylene. An article in the journal Science contains more detail.
“We found that the nanostructure really matters,” Notestein explains. “…This nanostructure is able to separate and sequence the reactions, even though both catalysts can do both reactions.”
Optimization of process conditions and independent tuning could yield further improvements, believe the researchers, but they will need the help of partners. The team is open to collaborating and discussing licensing or sponsored projects.
“Our reactors are not large enough to carry out experiments relevant for scaleup,” says Notestein. “This reaction is a tightly coupled pairing of an exothermic and endothermic reaction, and heat management will be an issue at larger scales. There are significant composition gradients down the reactor bed. These could be controlled and exploited through staged O2 addition, for example. With respect to composition, many platinum alloys are excellent for dehydrogenation,” he adds.
The porous overcoating structure stabilizes the Pt nanoparticles and pulls hydrogen toward selective H2 combustion for propane dehydrogenation reaction. Source: American Association for the Advancement of Science.
Making such nanoscale tandem catalysts on an industrially relevant scale poses few issues, Notestein stresses. “The core of our catalysts is entirely generic platinum-alumina. You can purchase these off the shelf at any scale. We use atomic layer deposition (ALD) to grow the indium oxide overcoat. … With careful optimization…the overcoats could be deposited by more conventional means,” he says.
The ALD coating also helps prevent sintering of the metal component. “I would not be overly concerned about hydrothermal stability or mechanical robustness; we actually use spherical, non-porous primary particles that should be fairly robust if formed up into pellets,” adds Notestein.
Ethylene and styrene production also could benefit from such tandem catalysts. “These two reactions are already commercial, or close to commercial, and an optimized catalyst based on this design might be well suited,” Notestein elaborates. “We hope to look more at ethane dehydrogenation as well as operating on simulated natural gas. We have other reactions that we will be examining with these and related catalysts. For example, we are initiating collaborations to use the intermediate H2 more productively.”
The team will next focus on validating their hypothesis about reaction mechanisms with in situ X-ray absorption experiments and kinetics studies to separately examine rates of dehydrogenation and hydrogen combustion. “Over the next year, we should be able to address most of the fundamental experiments,” concludes Notestein.