Pharmaceutical and fine chemical makers frequently rely on antisolvent crystallization, also known as precipitation crystallization, salting out or drowning out, to generate a solid from a solution in which the product has high solubility. The technique is used for a variety of applications such as polymorph control, purification from a reaction mixture and yield improvement.
Antisolvent crystallization achieves supersaturation and solidification by exposing a solution of the product to another solvent (or multiple ones) in which the product is sparingly soluble. The process can be semi-batch or continuous.
Although this technique has the potential to achieve a controlled and scalable size distribution, it's not without problems. The product requires purification or separation steps to remove the antisolvent(s). In addition, large changes in volume can hinder batch productivity and present agitation difficulties for minimum stir volume.
SEMI-BATCH OPERATION
Here, either the antisolvent is added to the product solution (normal addition) or the product solution is added to the antisolvent (reverse addition).
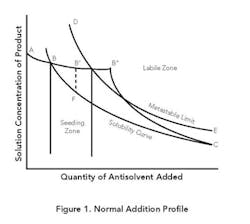
Normal addition. Figure 1 presents typical operating curves for this mode and a representative equilibrium solubility curve. The metastable zone (MZ) is the area between B-C and D-E. From point A to point B, antisolvent addition will proceed without crystallization because the solution concentration is below the equilibrium solubility. At point B, the solubility curve is reached. As antisolvent addition continues, supersaturation will develop. The amount of supersaturation created prior to nucleation is system specific and depends on the addition rate, mixing, primary or secondary nucleation rate, growth rate, feed location and the amount and type of impurities present in solution.
If the main goal is growth, the presence of a sufficient amount of seed and a slow antisolvent addition rate may allow the concentration in solution to remain completely in the MZ as crystallization proceeds. The closer the solution concentration profile is to the equilibrium solubility curve (B-C), the higher the possibility of achieving an all-growth process.
A system without seed or a fast addition rate can develop a high degree of supersaturation, which can result in rapid precipitation or crash out at point B″, in the labile zone beyond the MZ. Primary nucleation could be followed by continued nucleation and some growth (B″-C), eventually achieving equilibrium some time after all the antisolvent is added. If the concentration is allowed to go to point B″, the system also is subject to oiling out or agglomeration.
A common procedure for achieving growth while minimizing the possibility for seed dissolution is shown in curve B′-F-C. Antisolvent addition is stopped and seed is added at point B′, where the system is slightly supersaturated. (In situ measuring devices based on, e.g., Fourier transform infrared or ultraviolet can be used to determine when the concentration reaches point B′.) Also, the seed may be added in a slurry with the antisolvent starting before point B is reached to assure staying within the MZ.
Crystallization then is allowed to progress to relieve the supersaturation without the addition of more antisolvent (B′-F). Given enough time, the solution will closely approach the equilibrium solubility value (point F) while developing adequate surface area to primarily achieve growth during the addition of the remaining antisolvent. With this increased surface area and a sufficiently slow addition rate, the solution concentration can approach the equilibrium solubility for the remainder of the addition (F-C).
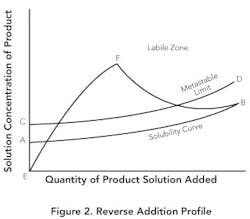
Reverse addition. If small particles are desired, it instead may make sense to add product solution to the antisolvent. Figure 2 shows this method. The solubility in the antisolvent is quite low at point A. Due to the low solubility, the supersaturation ratio will increase rapidly (E-F) before there's sufficient seed surface area to achieve any significant growth. If the addition is fast enough, growth will be minimized — resulting in a nucleation-controlled environment and formation of very fine particles. Even with slower addition and seed present, the low equilibrium solubility can create high supersaturation ratios throughout the addition and lead to the production of small particles.
A potential problem for all crystallization methods — but especially for reverse addition — is the tendency for organic compounds to oil out or agglomerate as fine particles into amorphous undefined structures. One possible cause of oiling out is that drops of the product solution are surrounded by the antisolvent, in which the solubility is very low, and this low solubility creates localized regions with very high supersaturation ratios. Before mixing to the molecular level is achieved, the localized high supersaturation forces the product out of solution without allowing sufficient time for ordering of molecules to enable crystal development. The resulting oily particles have a tendency to clump together before the occluded solvent migrates throughout the solution. As the mixture is aged, the oiled-out particles may transform into amorphous solids or become crystalline. Solids developed in this manner will likely have poor lattice structure.
Problems that may ensue include:
• large drops of coalesced oil that won't disperse and can harden into a gum/waxy material;
• agitation difficulties due to the size and physical characteristics of the wax;
• occlusion of impurities and solvent in the wax; and
• complications in downstream recovery and washing.
Another problem is that the fine particles may stick together. Strong agglomerates may survive intact but weak ones may reduce in size with continued mixing or subsequent handling such as pumping or centrifuging.
Clearly scaleup of reverse addition can be difficult due to the large deviations from equilibrium.
Seeding. As with all crystallization techniques, seeding may help avoid excessive nucleation. The seed can be added as a powder or in slurry form with the antisolvent. The latter often is preferred for ease of handling and reduced contamination. In addition, the seed can be conditioned via Oswald ripening.
Adding seed with the antisolvent offers an advantage over the traditional method of putting seed in at a single time — which poses the risk of adding too soon (seeds dissolve) or too late (nucleation has already occurred).
The limited solubility of seed in the antisolvent means a seed slurry can be prepared beforehand. This mixture normally will represent a small amount of the total antisolvent charge. It then is added near the saturation point until the MZ is reached. The antisolvent in this slurry reduces the solubility, causing the mixture to achieve supersaturation, which is relieved in the presence of the added seeds. The goal is to stay within the MZ, thereby promoting growth with limited nucleation via secondary mechanisms.
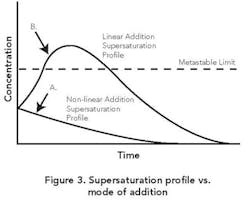
Addition strategies. Often addition takes place at a constant rate. This linear-profile procedure can yield a variable supersaturation whereby the MZ is exceeded early on, resulting in too much nucleation.
As Figure 3 shows, an initial slow addition rate followed by a gradual increase in rate can achieve a fairly constant supersaturation within the zone. This non-linear profile is analogous to the ones utilized for batch cooling or evaporative crystallizations [1]. The goal is to maintain the supersaturation of the solution consistently within the MZ while achieving constant relief of the same as growth on existing crystal surface area. As already noted, seeding techniques can help produce this desired outcome.
CONTINUOUS OPERATION
Some applications require a small mean crystal size and narrow size distribution. Examples include pharmaceutical materials requiring sub-micron or several-micron mean size where the active ingredient has marginal water solubility limiting bioavailability. Inhalation products also need these attributes. Making such products demands continuous processing via an in-line mixing device or a stirred vessel.
In-line mixing. At times this technique is used to obviate micronization or excessive milling. However, it can cause negative results such as dusting, caking, electrostatic charges and a polymorphic transformation.
In-line mixing equipment for crystallization includes impinging jets, vortex mixers, Y mixers and rotor-stator configurations. The antisolvent and product solution (which may contain seeds) are mixed in a very small active volume; this yields extremely high supersaturation values that are above the MZ, resulting in the production of a large number of nuclei. The two streams are mixed at the molecular level with excellent micromixing, with mixing times often being less than the nucleation induction time. Good control of nucleation can be achieved in the intensely mixed volume.
Impinging jets have high shear and high energy inputs in a small region and rapid localized intense mixing of the streams. A jet mixer can generate local energy dissipation rates ten times greater than those achieved in a stirred vessel.
Scaleup to commercial production from the laboratory or pilot plant often is feasible. Naturally, downstream recovery presents problems in terms of filtration, washing and drying.
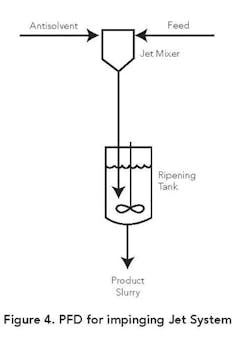
Figure 4 depicts a flow diagram for one type of impinging jet configuration. In this case the product is ripened in a stirred tank following contact of the product and antisolvent streams in the jet mixer. The ripening can be batch or continuous and is designed to facilitate diffusion of the trapped mother liquor in the nucleated solids. Adequate ripening time also is provided to convert amorphous solids into crystalline structures. In some applications seeds are added to the antisolvent stream or the ripening vessel.
Stirred tanks. When using such equipment, it's important to recognize that three types of mixing may impact product characteristics:
1. macromixing;
2. micromixing; and
3. mesomixing.
Macromixing relates to bulk blending in stirred vessels.
Micromixing determines the time of blending to a molecular level and the induction time for nucleation. It's influenced by impeller type and speed plus location of the antisolvent feed pipe. Mixing times will vary greatly — by a factor of more than 20 — throughout the vessel when operating at the same speed. Local energy dissipation rates can easily vary by over a 100 fold throughout the vessel. (Micromixing times can be an order of magnitude less for continuous in-line mixers.)
Mesomixing, in the context of this article, refers to the dispersion of the plume of antisolvent generated at the feed point as the solvent is added to the bulk solution/slurry. Without proper feed-point location and the right feed device/pipe, pockets of high supersaturation can occur, resulting in undesired nucleation. The time constant for mesomixing depends on the addition rate, feed point and diameter of the feed pipe. Too high a value can lead to premature nucleation.
Feed pipe location, pipe diameter and antisolvent flow can impact both micromixing and mesomixing times. A change in mean particle size and crystal size distribution (CSD) at different pipe locations would confirm product sensitivity to mixing.
Mesomixing can influence the product when the antisolvent feed rate is faster than the local mixing rate, resulting in a plume of highly concentrated antisolvent that isn't mixed at the molecular level. This can yield a high localized nucleation rate; the phenomenon can present scaleup difficulties, requiring a thorough engineering analysis for success.
The shortest mixing time constant occurs at the location of maximum turbulence in the vessel, which is just above the impeller for a down-pumping pitched-blade turbine (PBT), or at the point of discharge flow for a radial flat-blade agitator.
If the antisolvent is added in a poorly mixed zone such as at or near the surface or a baffle, a number of potentially undesirable results such as crash nucleation, oiling out or agglomeration may occur.
Subsurface addition of antisolvent at times can help avoid high levels of supersaturation and resultant nucleation when introduction is made at a zone of intensive micromixing. Results depend on the feed point location plus pipe diameter and antisolvent feed rate. For example, too large a pipe diameter could prompt a high supersaturation region prior to blending at a molecular level. Reverse flow with potential pluggage also could occur.
Genck [2] presents a detailed analysis of mixing in stirred tanks.
IMPROVING PERFORMANCE
Unfortunately, many industrial antisolvent crystallization operations are far from optimum. So, let's now look at a case history that illustrates a number of the challenges and some procedures that can mitigate them.
A drug maker was experiencing a problem in producing an active pharmaceutical ingredient (API). Crystallization gave poor product characteristics: an oily/waxy or amorphous solid, small crystals with high surface area (exceeding 30 m2/gm) that were difficult to filter and wash, and a small MZ. The company wanted to improve CSD, filterability and crystallinity.
The process took place in a fully baffled crystallizer with a 1.6-ft.-dia. 4-blade PBT operating at 60 rpm. The API was dissolved in isopropanol (IP) and crystallized by subsurface linear addition of isopropyl acetate (IPAc) for 1 hr. via a 2-in.-dia. pipe near a baffle. This led to a volume increase to approximately 1,000 gal. from the original 500 gal. No seeding was used. The slurry was aged at 20°C and cooled to 10°C.
Simulation using Visimix software [3] provided insights on the original operation as well as potential modifications. It showed that 60 rpm was inadequate, lacking in effective energy dissipation rates and having relatively high microscales of turbulence for dispersion of the antisolvent. In addition, the simulation indicated the characteristic micromixing time and mean time of circulation were high. Trial and error led to selection of 90 rpm and a modified procedure (Table 1).
The changes provided substantial improvements:
• Cake permeability increased by more than 100 times, resulting in easy filtration and washing.
• Mean particle size and CSD rose significantly.
• A highly crystalline product was formed.
• There was no evidence of breakage or extensive secondary nucleation.
As this points up, antisolvent crystallization can offer an attractive option but requires care in its implementation.
WAYNE J. GENCK, PhD, is principal of Genck International, Park Forest, Ill. E-mail him at [email protected].
References
1. Genck, Wayne J., "Better Growth in Batch Crystallizers," Chem. Eng., p. 90 (Aug. 2000).
2. Genck, Wayne J., "It's Crystal Clear, Part II — Scaleup, Simulation and New Technologies," p. 37, Chem. Proc. (Dec. 2003).
3. VisiMix, Ltd., www.visimix.com