Shelf drying is a common unit operation for reducing the liquid solvent content of solid cakes prior to material storage or downstream processing. Where the use of high temperatures could result in product stability issues, shelf dryers may employ vacuum to evaporate volatiles at low temperatures. Pharmaceutical manufacturing, for one, often requires efficient drying at less than ambient temperatures. The combination of shelf drying and sub-atmospheric pressure allows for efficient volatile material evaporation while maintaining the solid cake at relatively low temperatures. Determining the drying endpoint using in-process conditions that accurately predict the solid-cake moisture content minimizes the potential for a need to stop and restart the operation. We have found in a case study that the absolute pressure during a vacuum shelf drying operation correlates closely with the volatiles’ content of solid cake and, thus, can serve to regulate drying time.
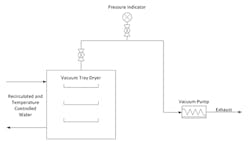
In this case, we must dry a crystalline solid product to a specified solvent content prior to storage and shipping for downstream processing. Technicians load a batch of wet solids that contain 20–40-wt.% solvent into stainless steel pans. The pans then are placed on jacketed shelves within the dryer and thermocouples are inserted into thermowells installed on the pans to measure cake temperature. The dryer is connected to a vacuum pump separated by a block valve that controls the start and end of drying. The vacuum pump runs at capacity throughout drying, with a flow rate of 40–82 acfm depending upon the operating pressure. The dryer shelf contains recirculated water that is temperature controlled to a specified set point to regulate heat transfer to the pans. Figure 1 shows the dryer and Figure 2 depicts the equipment layout.
[javascriptSnippet ]
Drying starts once the dryer door is sealed and the vacuum supply valve is opened. The shelf transfers conductive heat to the cake. Pressure is measured on piping connecting the dryer to the vacuum pump. The pump runs at full capacity, both removing solvent vapors and continually decreasing the pressure within the dryer. Initial pressure is as high as 7 mm Hg abs., but typically falls to 0.1–0.5 mm Hg abs. by the end of the drying operation. The thermocouples installed into the trays continuously monitor the solid cake temperature. It drops quickly at the start of drying from ambient temperature to between -10°C and -25°C as a result of evaporative cooling. The rate of evaporative cooling is greatest at the start of the drying operation when the cake has its highest volatiles’ content. Temperature soon levels off and then slowly increases for the majority of the process due to the ever-decreasing rate of heat transfer from the solids to the vapor as the drying rate falls. By the end of drying, the cake temperature typically is 5–11°C when controlling the jacket to 12°C.
Conventional Strategies
Control of the drying operation endpoint historically has been based on either a fixed time or a final cake temperature within a fixed time range. Both strategies heavily rely upon a defined time or time range to estimate when drying ends. A time-based approach poses inherent issues because factors that impact the drying rate vary from batch to batch and are difficult to control. The most influential factors are batch size or cake thickness, and initial moisture content.
Figure 1. In typical operation, ten pans containing product are placed on jacketed shelves with thermal couples inserted into wells on five pans.
Additionally, the temperature endpoint strategy has proven ineffective for several reasons. The level of leak rate into the dryer can impact the efficiency of solvent removal but is undetectable by the cake temperature alone. The placement of the temperature sensor within the cake also adds variability because the cake temperature changes as a function of its distance from the shelf. In addition, the cake isn’t uniform in initial moisture content, so a single-point temperature measurement within the cake isn’t always representative of the average of the entire cake due to lower or higher rates of localized evaporative cooling. These factors result in unacceptably high variability of endpoint moisture content.
In our case, prior to the vacuum dryer operation, the cake undergoes a forced nitrogen convection process that removes excess liquid solvent. As a result, the solvent contents observed in the process always start and end within the falling rate period of drying; therefore, the drying rate always is a function of the moisture content in the cake as mass transfer laws dictate. For this operation, batches enter the dryer with variable initial solid weights and liquid solvent contents. Batch size and the initial solvent content contribute to the volume of volatiles to be removed; larger or wetter batches generally require longer times to dry in the falling rate period of drying. Bigger batch sizes and, thus, thicker cakes also increase resistance to flow because diffusing vapor must travel a more torturous path to the surface. In addition, higher initial solvent content increases drying times because a greater ratio of initial solvent content to solid mass results both in a larger volume of solvent to remove and more-extensive evaporative cooling heat transfer occurring from the solids to the vapor. A higher extent of evaporative cooling removes heat from the solids and generates a lower temperature profile throughout the drying process, slowing the drying rate and extending the drying time.
Figure 2. The vacuum pump runs at capacity throughout the drying operation.
A Different Approach
We decided to try to determine drying endpoint based on product solvent content in the cake by indirectly gauging the vapor mass flow rate via pressure measurement. Assuming a low leak rate into the dryer, the pressure at any given time during drying is proportional to the volumetric flow rate as determined by the vacuum pump pressure/flow curve. In this case study, the vacuum pump capacity far exceeds the drying rate; therefore, the operating conditions all are on a sloped portion of the vacuum pump curve where volumetric flow rate continually decreases as pressure falls. Because the vacuum chamber is well sealed with a minimal leak rate and contains a vapor-producing stream from the cake in the falling rate period of drying, the pressure generated at any time reflects the mass flow rate of solvent vapor from the cake.
To check the assumption that the leak rate is minimal, we measured the pressure impact of the leak rate by running the vacuum dryer process without loading product. The pressure generated by the system at equilibrium primarily is a result of the system leaks, including from the vacuum pump. The leak rate pressure was found to be approximately 0.01 mm Hg abs. ±0.005 mm Hg. Because the pressure associated with leaking accounts for only 1%–15% of the total pressure at the endpoint condition, we felt comfortable treating the contribution of leak rate as minimal and relatively constant. These data support the conclusion that the large majority of flow through the system when drying stems from solvent vapor evaporative mass transfer from the wet cake, also identified as the drying rate.
The Ideal Gas Law enables calculating the drying mass flow rate from the measured pressure during drying, the volumetric flow rate as determined by the vacuum pump performance curve, and the average cake temperature. The Ideal Gas Law is a good approximation of the actual drying rate as a result of low operating pressures. The drying rate is required to correlate measured pressure and the cake solvent content. However, in this case study, calculating the specific drying rate wasn’t necessary because our desire was to predict the final volatiles’ content in the cake.
The correlation of drying mass flow rate to the actual cake solvent content in the falling rate period of drying is complex and depends upon several factors including the specific cake’s resistance to drying. The unknown geometry of interstitial spaces both between and within the solid particles making up the cake results in complex cake resistances. Therefore, empirical data are required to equate drying rate to a final volatiles’ content and complete the correlation to endpoint pressure.
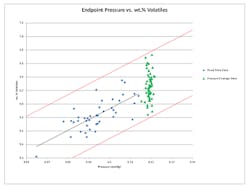
Figure 3. Pressure endpoint strategy enables accurate prediction of average volatiles’ content.
We experimentally determined the relationship between endpoint pressure and cake moisture content by measuring the final solvent content of the cake for 40 batches using a fixed-time drying endpoint strategy. We plotted the endpoint pressure and volatiles’ content (wt.%) of each batch on an x-y chart and found a linear correlation for the given data range (Figure 3). We used the best-fit linear relationship to approximate an average volatiles’ content that correlates to each endpoint pressure. We fit confidence limits of 99% (shown in red) to the data to show with high confidence the expected range of volatiles’ contents for each endpoint pressure condition. In this case, we selected a specific endpoint pressure to achieve an average solvent content of 6.25% while limiting the upper range to 6.8%.
Upon implementing this pressure endpoint drying strategy, we produced a set of 56 batches by controlling the endpoint pressure to 0.12 mm Hg absolute. The result was an average final solvent content of 6.26 wt.%, with a range of 5.85–6.73 wt.%, which closely resembles the targeted average and upper limit. Figure 3 shows that the average volatiles’ content with the pressure endpoint drying strategy was predictable by using a linear fit to the fixed time data. Additionally, the range can be predicted because the pressure endpoint drying data remained within the 99% confidence limits set for the fixed time drying data.
We now have implemented the pressure endpoint strategy; drying time (i.e., the time needed to reach the endpoint pressure) varies from -35% to +20% of the previous fixed time. Smaller and initially dryer batches require less time in the dryer while larger and wetter batches need more time. As a result of this strategy, the variability in the final solvent content in the cake has decreased by approximately 50%. These results support the conclusion that using a fixed pressure endpoint can compensate for batch-to-batch variability such as batch size (cake thickness) and initial moisture content.
STEVE WEBB is an associate consultant engineer for Eli Lilly and Company, Indianapolis, Ind. E-mail him at [email protected].