Heat Exchanger: Reject a Risky Rate Rise
This Month’s Puzzler
We use a lobe pump to move a sealant product from a reactor, after a conditioning heat exchanger, into a static mixer where we add 1 gph of stabilizer before sending the final formulation to the packaging fill line. The pump was sized for 35 gpm in a 3-in. Schedule-10 pipe. The operators claim they can get 40 gpm, sometimes 42 gpm, out of this pump. With some products, this occasionally is true. Now, management wants to increase the capacity of the fill lines to 40 gpm by taking advantage of this higher flow rate. Part of the plan calls for raising the exit temperature of the conditioning exchanger to get more flow. The research group is readying new products for introduction next year; some have higher viscosities than the current line of sealants. This flow increase seems like a bad idea to me. Am I wrong?
Battle The Idea
The reason the operators get more flow out of the pump is because pressure drop calculations assume the fluid is Newtonian. Rarely are liquids completely Newtonian but the assumption makes calculations easier because the viscosity of a Newtonian liquid is constant at a given temperature. Thixotropic liquids exhibit a decrease in viscosity with an increase in applied force. Another issue is whether the liquid is organic or water-based. Don’t expect much greater flow with water-based mixtures, which usually act like Newtonian liquids and so maintain a nearly constant viscosity. If the viscosity drops the flow rate rises for a given pressure. This is what the operator sees.
Increasing the temperature is a bad idea. The purpose of this change is to lower the viscosity to boost the flow rate to the filler lines. The problem is that the fill lines count on that viscosity. If the viscosity drops too much the flow from the filling lines will be too fast, causing spills and waste. In addition, product lines count on viscosity to control leaks, so there will be a mess in the packers.
Another concern with raising the flow temperature is the potential for greater oxidation of the product. The higher temperature can lead to unexpected reactions and discoloration. Often tanks holding organic ingredients rely on a nitrogen purge to reduce O2 exposure.
There are other problems to consider, as well.
I’m assuming all the products— including the new ones being developed by research — have similar rheology. Nevertheless, I recommend testing; most pump manufacturers will characterize liquids, both to add to their library and to help customers. Obviously, you must make arrangements to protect proprietary formulation details.
So, what can be done to make this project feasible? To start, collect properties on all liquids. In addition, either raise storage capacity between the filler and the static mixer or boost the capacity of the lobe pump. If the pump was sized correctly in the first place, an increase may be as simple as replacing the motor or the gearbox. You will want to consider the effect of higher pump speed on the properties of the product and greater flow resulting from thixotropic decrease in viscosity.
Lastly, build a hydraulic model. Most software relies on the Darcy-Weisbach equation, which is strictly limited to Newtonian fluids. You can get a conservative model by using equivalent lengths instead of the Crane fitting K factors in the equation. Putting more flow through a pipe raises the pressure at the discharge of the lobe pump; so, check that the pressure still remains below the set points on the relief valves. Perhaps you can justify replacing a section of the pipe network to reduce the pressures and increase the allowable flow — without blowing the reliefs.
Dirk Willard, process engineer
A&B Process Systems, Stratford, Wis.
May’s Puzzler
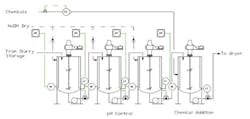
We use a bank of continuously stirred reactors to raise the pH of an inorganic slurry in preparation for drying and packaging (Figure 1). The proper pH is critical for controlling dryer corrosion, which would result in rust contamination. Some dryer chemicals are pH-sensitive and the pH of the product must be between 6.7 and 7.9. We add finishing chemicals for packaging in the last tank. The process is fraught with problems. The pH probes in the beginning tanks foul often with different campaigns of product. Sometimes the chemicals added in the last tank result in product rejects in packaging. How can we improve this process and reduce product rejections?
Figure 1. The first three tanks control pH while finishing chemicals are added in the last tank.
Send us your comments, suggestions or solutions for this question by April 13, 2015. We’ll include as many of them as possible in the May 2015 issue and all on ChemicalProcessing.com. Send visuals — a sketch is fine. E-mail us at [email protected] or mail to Process Puzzler, Chemical Processing, 1501 E. Woodfield Rd., Suite 400N, Schaumburg, IL 60173. Fax: (630) 467-1120. Please include your name, title, location and company affiliation in the response.
And, of course, if you have a process problem you’d like to pose to our readers, send it along and we’ll be pleased to consider it for publication.