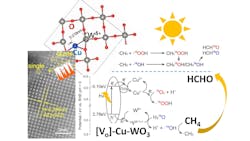
A novel catalyst derived from tungsten trioxide has achieved near 100% conversion of methane to formaldehyde under 420-nm light irradiation and at room temperature.
Researchers from chemistry and chemical engineering departments at University College London, the University of Hong Kong and Tsinghua University, Beijing, China, collaborated to develop the material, which features a dual-active site comprising copper and tungsten atomic species that work in tandem to ensure an effective and selective conversion process.
Mechanistic analysis found that the tungsten trioxide substrate provides the visible-light responsive activity for methane conversion, while the atomically dispersed copper acts as an effective electron acceptor.
“Further research will be focused on improving the catalyst’s efficiency and selectivity,” said Zhengxiao Guo, professor of chemistry and mechanical engineering at the University of Hong Kong and corresponding author of a recent paper describing the work in Nature Communications. “Tasks include enriching the atomic active sites during catalyst synthesis and scale-up and to design innovative reactor systems, for example, to enhance solar/catalyst interactions and to achieve multi-pass reactions.”
Guo also notes that the dual-active site strategy developed here can be applied to other chemical reactions that involve multiple electrons to complete the process, such as water-splitting hydrogen generation and carbon-dioxide conversion into other chemicals.
“It extends the scope and flexibility for fine-tuning of the local microenvironment, so that reaction rate and pathways can be tailored to maximise productivity and improve selectivity,” he added. We are applying similar design strategies for other chemical reactions — with very promising discoveries in the pipeline.”
In terms of commercial potential, Guo says that one of the key challenges is to control the synthesis conditions and to tailor them for large reactors in scale-up reactions so that reproducibility is maintained for the catalyst performance. “Another challenge is to improve further the durability of such catalysts,” he said. “Both are also common issues with catalysts in general. We are working on ways of tackling those.”
The team is actively seeking industrial partnerships for continued development of the catalyst system and its practical applications. Guo says it is important to note that even the low concentrations of formaldehyde generated during the reaction can be applied in household, commercial and medical products. “Such low concentrations can be readily produced and applied on demand and on site, offering great flexibility for product design in the supply chain,” he concluded.
About the Author
Seán Ottewell
Editor-at-Large
Seán Ottewell is a freelance editor based in Ireland. He has an impressive background in the chemical industry. After earning his degree in biochemistry at Warwick University, UK, he earned his master's in radiation biochemistry from the University of London. His first job out of school was with the UK Ministry of Agriculture, Fisheries and Food, London, where he served as scientific officer with the food science radiation unit.
From there he entered the world of publishing. In 1990, he was the assistant editor of The Chemical Engineer, later moving on to the chief editor's position. Since 1998, he has been a regular contributor to European Process Engineer, European Chemical Engineer, International Oil & Gas Engineer, European Food Scientist, EuroLAB, International Power Engineer, published by Setform Limited, London, UK.
Chemical Processing has been proud to call Ottewell Editor at Large since 2007.
He and his family run a holiday cottage in the small village of Bracklagh in East Mayo. He also fancies himself an alpaca farmer.