How Small Can You Go?
Advances in powder-based processing long have been focused on reducing particle size and improving the particle uniformity. Nanosized powders ," particles at the nanoscale, or approximately 1 nanometer (nm) to 100 nm in size ," are becoming increasingly critical to innovations in numerous applications, including catalysis, coatings, cosmetics, electronics, sensors and drug delivery.
Nanopowders offer controlled functionality, increased reactivity and a number of other advantages over existing materials. Moreover, new powder production and synthesis methods promise to further improve particle capabilities and push particle sizes to new lows.
Emulsion aggregation
Xerox has reinvented toner production through its emulsion aggregation (EA) process, which creates 1 micron (m) to 15 m particles from smaller nanometer-sized particles. The company also said the technology has potential applications in biotech, personal-hygiene and related applications.
Pat Burns, manager of the composite and nanostructure materials area at the Xerox Research Centre of Canada in Mississauga, Ontario, says the technology sprung out of the company's desire to make smaller toner particles in an economical manufacturing process. Xerox wanted to control particle size and shape to the greatest extent possible.
"The smaller particle size will give you improved image resolution, and you'll end up using about 40 percent less toner on a page," explains Burns. "We wanted to control structure ," to be able to put different things inside, put wax particles inside, not on the surface."
Xerox also wanted to be able to switch to lower-melt polymers, notes Burns. "With conventional processes, you're limited to certain resins," she says. "They have to be brittle enough to break in the grinding process."
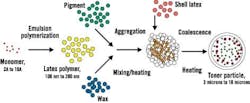
The environmentally friendly EA process starts with monomers, says Burns, and uses emulsion polymerization to grow tiny polymer particles in an aqueous environment. The toner particles then are combined with pigment particles and wax particles, which are also in water and very small. See Figure 1.
Figure 1. Emulsion Aggregation Toner Process
The EA process consists of a stepwise water-based approach for controlled particle growth.
Source: Xerox.
"Basically, we pour them together," Burns explains. "It's not very viscous; [it's] like water. Then we add a reagent to cause the particles to flocculate. Basically, most of the particles are stabilized with a negative charge, so we put in a positively charged material. That causes destabilization, and it causes the particles to flocculate together."
By controlling the physical chemistry of the mixture, says Burns, Xerox can determine the manner in which the particles form. "Basically, we control the temperature, time and stirring," she says, "and obviously the amount of flocculent we're adding. That causes the particles to come together in a controlled fashion."
After halting particle growth using an anionic stabilizer, Xerox ends up with a mixture that has a very narrow particle size distribution. That mixture then is heated, says Burns, so the polymer resins start to flow and form one solid particle. The material then is cooled down.
"What we end up with is toner particles that are in water," Burns explains. "There are some reagents that we use to make the particles in the water with them ," then we have to isolate them. What we do is washing, essentially just filtration. You end up with toner particles that are kind of the consistency of wet sand. Then you have to dry [them]."
The types of polymers used in the EA process allow the presence of functional groups on the surface of the resulting microspheres for potential ligand attachment or chemical modification, notes Xerox. The process permits magnetite, colorants and other materials to be incorporated within the microspheres and also enables the creation of layered structures.
Xerox introduced a toner product based on the new process about four months ago in Spain and plans to bring it to the United States as well. The company currently is offering the technology to parties interested in licensing it for use in biomedical, personal-hygiene and other applications. For more information, contact Ed Francis, licensing executive for Xerox, at [email protected].
Laser vaporization
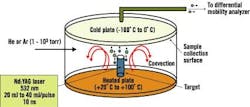
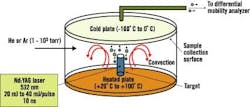
Dr. Samy El-Shall, a professor of physical chemistry in Virginia Commonwealth University's (VCU) Department of Chemistry, says VCU researchers have been perfecting processes that use laser vaporization to synthesize nanoparticles of controlled sizes. See Figure 2.
Figure 2. Laser Vaporization Controlled Condensation
Shown here is the experimental setup for nanoparticle synthesis using laser vaporization in a convective atmosphere.
Source: Dr. Samy El-Shall, Virginia Commonwealth University.
Researchers start with a bulk alloy material, says El-Shall, or make a specific target based on the composition desired. "These are large particles, 100 m," explains El-Shall. "We take the different composition we want to make from this powder, mix it and compress it under high pressure to form a target."
The target then undergoes laser vaporization. "We call it a tailored target," explains El-Shall. "The number of atoms [the laser] will reduce will be proportional to the composition that you have in the target."
The target is vaporized into metal atoms that will tend to form nanoparticles, says El-Shall. "When [we] vaporize, we don't want them to diffuse," he emphasizes. "We want [the atoms] to condense under our conditions before they go away from each other. So, in this case, you form new particles from the condensation of these atoms, and these new particles will not be just iron, just aluminum and just nickel ," they will contain three atoms in the composition we desire."
The composition often is not precisely the desired composition, El-Shall stresses, so researchers adjust it through trial and error. "It really depends on the physical properties of the metal atoms and the diffusion coefficient," he notes. "Sometimes if you have two different metals with very different properties, one diffuses several ," or 100 ," times faster than the other. In this case, you have a problem ," they will not be together at the same place at the same time. You can compensate for that in the composition. Sometimes we have to start with something like 80 percent of A and 20 percent of B to get the nanoparticles 50/50," he adds.
Catalysis is one potential candidate application for these nanopowders, notes El-Shall. A silica platinum composed of nanoparticles showed "very strong catalytic activity for hydrolyzation reaction, he adds.
Such nanoparticle-based catalysts are heterogeneous catalysts, says El-Shall, but work much like homogeneous catalysts ," sometimes even better ," because the particles are so small. "A homogenous catalyst is very well dispersed because it's contained in the reaction medium," he explains.
El-Shall and other VCU researchers recently announced a nanoparticle breakthrough using the laser vaporization process. They assembled a new class of metallic and related types of nanoparticle fibers and filaments by suspending nanosized pieces of metal and other materials in vacuum chambers filled with electrically charged vapor.
"Using a process that produces ultra-pure nanoparticles with laser vaporization and controlled condensation and then applying an electric field, we have shown that several classes of metallic and semiconductor nanoparticles can be assembled into chains and filaments that retain their unique properties," says El-Shall. "This holds great promise for the development of novel functional materials and for the engineering of a wide variety of nanodevices and sensors."
The discovery could result in the development of stronger plastics that incorporate nanoparticle filaments within polymer chains, added El-Shall. Researchers now are evaluating the nanoparticles for use as catalysts to remove carbon monoxide from air, as well as for use as a "smart dust" that detects chemical and biological warfare agents and environmental pollutants.
Understanding nanoparticle fluidization
Current nanopowder research is not limited to innovative production routes. Researchers at the Illinois Institute of Technology (IIT) in Chicago and the Newark, N.J.-based New Jersey Institute of Technology have been working to understand the physics of fluidization and transport of nanoparticles under a National Science Foundation (NSF) grant. A better understanding will advance the production of nanocomposites with tailored properties for catalyst and other applications.
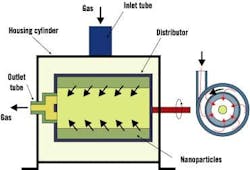
Dr. Hamid Arastoopour, Max McGraw professor and chairman of IIT's chemical and environmental engineering department, said researchers want to get a handle on nanoparticle behavior within conventional, rotating and circulating fluidized beds. Fluidization is used widely in many powder processes within the chemical, petrochemical and pharmaceutical industries, but on micron-scale particles. These applications could benefit significantly from the unique properties of nanoparticles. Figure 3 shows a rotating fluidized bed in a nanoparticle operation.
"With a nanoparticle, the size is very small, and with a little bit of force or if you blow the air through or fluid through, the particles are airborne, and they will leave the reactor," Arastoopour explains. "It's difficult to keep them because they're extremely small and very light. We are increasing the gravitational force ," in that way, we keep the particles inside the reactor. Then we can fix them and put them in the catalyst and so on."
Researchers increase the gravitational force on the reactor and then rotate the reactor, says Arastoopour. "We call it rotation of a fluidized bed," he says. "The centrifugal forces will create this kind of force pushing them toward the wall. Therefore, we can go up to 50 G.' The particles, although they're very small and light, [stay] in the reactor."
By changing the flow of the gas or fluid, researchers can alter the size of the aggregate of the smaller nanopowders, says Arastoopour. They then can control the surface area. "We can have a much higher surface area, enhanced conversion," he maintains. "Therefore, we can have much smaller reactors and more efficient reactions." Operation costs also are reduced, he adds.
During the four-year NSF project, researchers hope to come up with a computer simulation that uses a governing equation for the design of systems using nanoparticles. Also planned, says Arastoopour, is a very detailed experimental and theoretical analysis of the high-gravitational-force rotating fluidized bed.
"At the end of this project, we hope that scientists and engineers who want to use reactors based on nanoparticles [can benefit from] tools for design and calculations," Arastoopour stresses.
Figure 3. Rotating Fluidized Bed
A rotating fluidized bed is used to determine the effect of varying gravitational forces on fluidization behavior.
Source: Dr. Hamid Arastoopour, Illinois Institute of Technology.
Thinking small
Outside academia are numerous small companies actively involved in nanopowder research. Two such companies are Nanopowder Enterprises Inc. of Piscataway, N.J., and Tetronics Ltd. of Faringdon, Oxfordshire, England.
Nanopowder Enterprises currently is performing research in the coatings area, working with nanopowders to produce harder, scratch-resistant products.
"We provide a mechanism [that imparts] multiple functionality to the coating," says Dr. Ganesh Skandan, chief operating officer of Nanopowder Enterprises. For example, he says, a coating could be transparent, hard, scratch-resistant and conductive.
The 12-person company also is developing "nano-additives" for coolants. "If you put nano-additives in," says Skandan, "they alter the thermal properties of the fluid." If the thermal conductivity is increased, he adds, the size of the cooling equipment potentially could be reduced.
Nanopowder Enterprises also has its hands in other nanopowder applications. However, Skandan maintains that the company's mission ultimately is "to be a nanomaterials provider" ," a manufacturer providing products directly to the end user.
At Tetronics Ltd., the principal focus is the application of thermal plasmas either as a clean heat source or in material processing. According to Dr. Michael Wise, a Tetronics process engineer, the company has been producing nanopowders and other novel materials for more than 15 years.
"The production of nanopowders involves the vaporization of the feedstock material in a plasma flame," explains Wise, "then recondensing the vapor, with or without the use of a quench gas, and then collecting the resultant powder."
This route results in contaminant-free spherical particles that can be formed from solids and faceted particles. The powders have very high specific surface area/mass ratios and potential uses in electronics, catalysis and other applications.
Plasma flame temperatures typically range between 5,000 Degrees F to 14,000 Degrees F for powder production, says Wise. "At these temperatures, even refractory oxides can be dissociated into their constituent elements," he notes, "which results in the feedstock being transformed into a homogeneous gas phase with the potential for rapid chemical reaction."
The company has commercialized two nanopowder-related processes, says Wise. The first is used for the production of oxides, including mixed oxides and doped oxides. The second process, says Wise, is used to produce an "energetic" aluminum powder for rapid-burn applications such as rocket fuel he adds.
In the future, Wise expects nanopowders to become more widely used as catalysts and says plasma-induced powders offer great potential in this area. "Plasma-produced powders tend to have higher surface activities," he maintains.