Choose The Best Measurement Option To Optimize Ethylene Production
Ethylene is one of the most valuable building blocks in the petrochemical industry, making purity a high priority for producers. To ensure top quality, ethylene plants must deploy highly responsive control technology. In recent years, some critical segments of these plants—such as ethylene fractionation, acetylene control and final product certification—transitioned to laser-based process gas analyzers as a better measurement option over gas chromatographs (GCs).
GCs are still a good option in the “hot” sections of the process, while laser technology is more appropriate in the cold section of the process, particularly in ensuring purity measurements are reliable and accurate.
Ethylene Fractionation Challenges
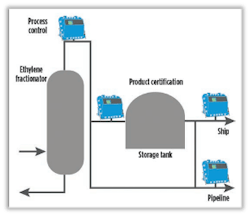
End-product purity of 99.99% is the goal of every ethylene plant in the final purification step, which happens in an ethylene fractionation tower (Figure 1). To ensure on-spec production and meet the level of purity required, reliable gas analysis is critical.
Differentiating between the different process media in the fractionator isn’t always easy, especially as ethylene and its precursor ethane, for example, have similar physical properties, making them difficult to separate. Ethane gets separated and recycled back to the process, while the ethylene product leaves the fractionator overhead.
Maintaining ethane close to the specification limit is part of maintaining a fine balance within process control to make sure the correct purity of ethylene is reached and maintained. The intent is to avoid going off-spec or unnecessarily recycling ethylene, which can result in substantial reprocessing costs and excess energy usage.
The required measurements in the fractionator must be made quickly and accurately to optimize real-time process control. Laser-based measurements help satisfy this requirement by providing updates every few seconds, whereas GCs may take several minutes.
Real-time Process Control
GC performance is more than adequate in many ethylene purity applications, where speciation of heavy gases is more important than fast response. But when instantaneous, continuous measurements of smaller molecules are required in critical areas of the purification train and for product certification, other technologies, such as laser-based measurement methods, may be more appropriate.
The detection capabilities of laser-based gas analyzers vary depending on the type of lasers employed. Lasers are semiconductor devices that produce monochromatic light, which traditionally has been mainly in the near-infrared wavelength range. They use tunable diode laser (TDL) technology, which allows only limited detection coverage of one or two gas components.
However, with the introduction of quantum cascade laser (QCL) technology, the spectral coverage has been extended to cover the valuable mid-infrared part of the electromagnetic spectrum, giving access to a wider spectrum of gas molecules. Detecting mid-IR wavelengths using QCL is advantageous because it enables gas analyzers to detect multiple gas components.
Also, some next-generation hybrid gas analyzers combine both TDL and QCL lasers, extending detection coverage over both the near- and mid-infrared range to sense many gas species simultaneously using a patented laser chirp technique, which provides instantaneous frequency sweep through many gas analytes of interest.
The duration of the laser chirp is about one microsecond, scanning a spectrum of one to three wavenumbers. Each laser is selected to measure one or two process components of interest within the specific spectral window, producing a unique absorption spectrum. This type of analyzer can house up to six lasers and measure up to 10 gas components, eliminating the need to deploy multiple analyzers.
The analyzer’s software converts this absorption spectrum to a real-time concentration measurement by using the fundamental principles of the Beer-Lambert law. These analyzers also can provide ongoing diagnostic and health checks of the measurement. They’re also sensitive enough to measure trace levels of gas components.
With the lasers capable of chirping at a frequency of up to 100 kHz, it becomes possible to gather real-time validation of measurements, allowing for automated decision-making and more proactive process-control practices. The availability of detailed multi-component data is particularly useful when looking to certify ethylene purity by enabling faster certification.
In addition, laser-based measurements are continuous, which minimizes the potential for process upsets, such as over-cracking of hydrocarbons in acetylene control—an issue that can cost hundreds of thousands of dollars per hour.
Advances in Spectroscopy
Historically, one of the challenges when making these types of high-sensitivity measurements with laser absorption spectroscopy has been the interference of atmospheric gases. This is a particular problem when determining ethylene product purity because the gases under scrutiny, such as CO2 and H2O, are also present at a significant level in ambient air.
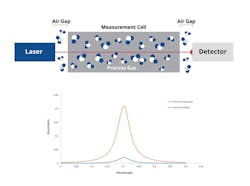
The specific readings are compromised by the external path that the laser beam travels through an optical system before reaching the process sample within the measurement cell (Figure 2). That external light path adds an unwanted contribution to the spectrum measured by the laser, especially where strong absorption lines are being targeted for measurement. This means the higher the sensitivity of the measurement, the more likely the external light path can compromise the readings.
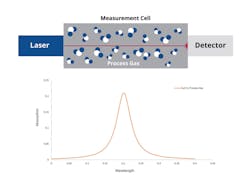
However, in recent years a new approach has been developed to nullify this effect. This zero-gap design eliminates the external light path for laser measurement by close-coupling the lasers and detector to the measurement cell (Figure 3).
Zero-Gap Design Details
Implementing a zero-gap measurement methodology (Figure 3) is possible when the external path spectral contribution is eliminated by reducing the laser beam’s atmospheric exposure to near zero. This approach uses a mechanical and optical design that eliminates any contribution to the spectrum arising from a laser-beam path external to the measurement cell. This design enables detection of sub-ppmv of H2O, CO2 and other gases present in the atmosphere, without the requirement to purge the analyzer housing.
Figures 2 and 3 represent the difference graphically. For clarity, these schematics have been simplified by removing some of the additional elements that would normally be found in the optical design, such as steering mirrors or lenses.
Figure 2 shows the standard analyzer layout where the laser beam travels through ambient air prior to and after the measurement cell. Figure 3 shows how the zero-gap analyzer is set up to keep the laser radiation inside the process gas sample of interest with minimal, if any, of the light path going through the atmosphere.
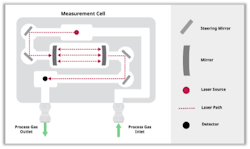
The innovation is in the mounting and positioning of the steering mirrors inside the sample cell, allowing the lasers and detectors to be positioned directly adjacent to the cell, effectively eliminating any traversal of the laser light through open air (Figure 4). As with the standard practice of allowing sample cell mirrors to be in contact with the process gas to achieve extended pathlength configurations, the optics use robust materials specifically designed for extreme applications to ensure measurement reliability.
A zero-gap analyzer configuration can combine up to six QCLs and TDLs in a single system for gas measurement (Figure 5). This hybrid laser system uses identical laser modules for QCLs and TDLs, and two detectors can be installed in the system to broaden insight and allow detection of the mid- and near-infrared light, depending on the types of laser installed.
In the zero-gap configuration, the laser light’s total path length outside the cell assembly is reduced to less than one mm. Compared to a traditional analyzer with an external path of about 0.5 m, this is a reduction by a factor of 500 in the light path external to the sample cell, minimizing spectral absorption from the external atmospheric gases.
Laser Technology in Practice
As a case in point, a European chemical company transitioned to QCL-based measurement from GCs as part of an upgrade to their ethylene purity monitoring systems. The operators needed a solution that could deliver measurement of various gas analytes without the deployment of multiple analyzers. A multi-component, QCL- based analyzer measurement was used to ensure ethylene product quality through real-time impurity monitoring of key analytes—including CH4, C2H2, CO, CO2, and C2H6 (product used was Rosemount CT5800 Continuous Gas Analyzer from Emerson.)
The analyzer was able to offer highly sensitive CO2 measurement with a 0-5 ppm range and sub-ppm Limit of Detection of 0.05 ppm. The design has also helped eliminate interference from ambient CO2 levels, delivering highly accurate measurement and ensuring on-spec ethylene production.
Other Applications
Laser-based analyzers are also useful in other areas of ethylene production. For example, a North American plant recently used an Emerson continuous gas analyzer to monitor C2H2, C2H4 and C2H6 through the acetylene converter feed and mid-bed units, with critical measurement of the acetylene levels transitioning from % to ppm levels when traversing the converter.
This plant also employed QCL technology in the same ethylene production plant for a different type of purity application, in this case not for monitoring of ethylene product, but the purity of hydrogen produced from onsite pressure swing adsorption units. CO was the impurity of interest in this application, and it was measured down to a 0.05 ppm limit of detection and over a range of interest of 5 ppm, providing the data necessary for real-time process control.
Design Considerations
GCs are suitable whenever there is a need to measure heavy hydrocarbon gases above C2, particularly when these are present in a complex mixture of several hydrocarbons, and when speciation is more important than fast response. This makes GCs a better fit in many “hot end” applications, such as optimization of furnace operation.
When measuring some heterogeneous diatomic molecules such as hydrogen, GCs are more appropriate because laser-based analyzers making measurements in the infrared range are not capable of providing hydrogen analysis. For oxygen measurement, laser-based continuous gas analyzers can be used, depending on the required detection limit.
About the Author
Beth Livingstone
Beth Livingstone is the global product manager for the quantum cascade laser process gas product line at Emerson. Beth holds a Master’s Degree in Chemistry and a PhD in Physical and Theoretical Chemistry, both from the University of Oxford.