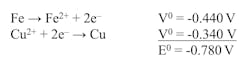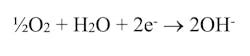Cooling Towers: Managing Galvanic Corrosion and White Rust Formation - Part 4
Key Takeaways
- Electrochemical potentials determine corrosion behavior: Metals with lower (more negative) half-cell potentials than hydrogen readily corrode in acidic environments, while noble metals like copper, silver, and gold resist attack without strong oxidizers.
- Galvanic and localized corrosion depend on material pairing and environment: Contact between dissimilar metals, manganese oxide deposits, or poor passivation can create anodic and cathodic sites that accelerate damage, particularly in cooling systems.
- Corrosion rates increase with conductivity, temperature, and acidity: Higher dissolved solids, warmer surfaces, and lower pH all enhance electrochemical activity, underscoring the need for careful pH control, materials selection, and surface conditioning in system design and operation.
Editor’s note: The previous installment in this series examined several of the most important corrosion issues in cooling water systems, including conditions that can lead to pitting, a very insidious mechanism. This part continues the corrosion discussion with a primary focus on two other important issues: galvanic corrosion and white rust formation in towers with galvanized steel components. Each can greatly reduce the longevity of cooling water equipment.
Different metals vary in their tendency to release electrons in electrochemical reactions, which directly affects their corrosion behavior. Standard reference tables quantify this chemistry. One straightforward example is metal behavior in acidic solutions, where the hydrogen ion (H+) half-cell reaction, by definition, is given a potential of 0.00 V for a 1 molar H+ solution.
2H+ + 2e– ⟷ H2
Table 1 illustrates the half-cell potentials of several common metals as compared to the hydrogen ion potential.
Important concepts that the table illustrates include:
- The metals above hydrogen will corrode in mineral acid solutions.
- Copper and its alloys are on the "noble" side of the hydrogen potential, meaning that the alloys usually remain stable in mineral acids. Oxidizing agents are needed to solubilize these metals.
- Silver, platinum and gold are three of the truly noble metals. For highly inert metals like gold, special solutions, such as aqua regia, are necessary for dissolving metal.
A strong driving force exists for this reaction.
Per changes to open-recirculating cooling water chemistry in the 1980s, which will be highlighted in Part 5, most cooling systems operate at a slightly alkaline pH. The typical cathodic reaction in these environments is:
The half-cell potential of this reaction is 0.401V.3 So, copper alloys, which are not oxidized by dilute mineral acids, can be oxidized by dissolved oxygen. A particularly aggressive environment is one containing dissolved oxygen and aqueous ammonia, where the D.O. generates a surface layer of cupric oxide, CuO, that is then gradually dissolved by the ammonia. This is a common reaction on the steam side of power-plant surface condensers tubed with copper alloys.
For the corrosion shown in Figure 1, a dielectric union to break the physical contact of dissimilar metals is a common solution. A broader concept involves the physical ratio of anodic to cathodic surface area. One example, often presented in literature, is the use of steel rivets to attach copper alloy plates that then are placed in an aqueous environment. This is a classic case of small anodes in a large-cathodic field, which can lead to rapid failure of the steel. The reverse situation, copper-alloy rivets holding steel plates together, spreads the galvanic current over a large anodic field, greatly reducing localized corrosion.
A galvanic issue that has caused difficulties in some locations is manganese-influenced pitting, with many problems reported along the Ohio River in the United States. Dissolved manganese in cooling water can be oxidized to manganese dioxide (MnO2) by chlorination for microbiological control. MnO2 forms a thin, varnish-like coating on heat-exchanger surfaces (Figure 2).
The deposits are strongly cathodic to the underlying metal and can cause severe localized corrosion, especially during periods of chlorination. 304 and 316 stainless steel are very susceptible to galvanic manganese corrosion, but it can also affect admiralty brass, aluminum brass and cupro-nickel.
White Rust
Many commercial cooling towers of moderate size are fabricated from galvanized steel. Galvanized components may be part of larger towers, even if the main support structure is fiberglass reinforced plastic or wood. For systems containing galvanized components, a conditioning process is needed to ensure that the galvanized materials develop a proper passivation layer.
If this conditioning is not performed, or if subsequent operation raises cooling water to an elevated pH, zinc will react with water to form a porous zinc hydroxide layer, which then reacts with carbonate and converts the material into a non-protective deposit known as “white rust” (Figure 3). The deposits are characterized by white, fluffy or waxy accumulations that adhere to galvanized steel surfaces and provide no protective barrier. If unaddressed, white rust can become very destructive.
Methods to prevent white rust in new towers include the use of an inorganic phosphate passivation program using a minimum of 100 ppm calcium as CaCO3 and 400-450 ppm [orthophosphate] PO4 and operating for 45-60 days with cooling water in the pH range of 7.0-8.0. This treatment regimen forms non-porous zinc carbonate/zinc hydroxide surface barrier.
For surfaces properly conditioned, the following table from a major cooling tower manufacturer outlines recommended operating guidelines.
Below are several other important cooling water corrosion mechanisms:
Crevice corrosion: Crevice corrosion develops at the crevices of mechanical joints, such as flange gaskets, the rolled ends of tubes or at other locations, including the boundaries of deposits. These locations can become oxygen depleted and develop into anodes that initiate localized corrosion, similar to pitting.
Erosion corrosion: Fast-flowing fluids, fluids that contain abrasive particles and changes in flow direction can induce erosion corrosion. Materials selection during the project design phase is important for minimizing erosion. For example, admiralty brass, which has excellent heat-transfer properties, is a relatively soft metal. Raw water containing suspended sand particles can degrade the material rather quickly. Cavitation is a particular type of erosion corrosion that most commonly affects pump impellers. Without sufficient head pressure at the pump suction, air bubbles in the water may collapse. This effect can generate large, localized forces that damage the metal.
Stress corrosion cracking (SCC), corrosion fatigue and intergranular corrosion: Nearly all metals, except for highly specialized applications, comprise small crystals or grains. Induced and internal stress can cause cracking along grain boundaries (intergranular) or sometimes across grains (transgranular) during service. These mechanisms are complex. Constituents in the water, such as oxygen and chloride, can significantly accelerate SCC. Field welding may cause stress and influence the metallurgical chemistry in heat-affected zones. Process fluid temperature has a large impact on SCC in some metals, with susceptibility increasing rapidly with increasing temperature. Corrosion fatigue may be a major problem in equipment that frequently cycles up and down in load.
Additional information on these corrosion mechanisms is available in the references.
Influences on Corrosion and Corrosion Rates
Numerous factors may influence the severity of corrosion or the corrosion rate. Some of the most important include:
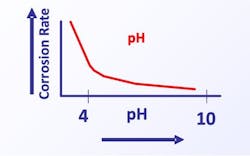
pH
Figure 4 illustrates the general effect of acidity/basicity, as measured by pH, on carbon steel.
The graph clearly shows the decrease in general corrosion with increasing pH. This influence served as a guiding principle when acid-chromate scale/corrosion treatment programs were replaced with phosphate-phosphonate programs in the 1980s, as will be outlined in Part 5.
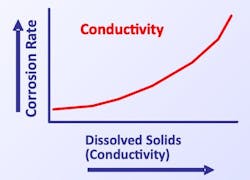
Conductivity
Increased dissolved solids concentration and corresponding solution conductivity enhances the electrochemical circuit potential.
Temperature
Chemical reactions typically accelerate as temperature rises. As a general estimate, reaction rates tend to double with every 10°C temperature increase. These general guidelines also apply to corrosion chemistry. Another point to keep in mind, which can also affect the potential for scale formation, is that the surface or “skin” temperature of components like heat exchanger tubes and plates can be significantly greater than the bulk fluid temperature. Reactions may speed up or become more severe at hot surfaces. Other factors that influence corrosion potential include fluid velocity, surface imperfections, disruptions of metal structure and chemistry at weld locations. Additional details are available in the references below and from the Cooling Technology Institute.
Next: Addressing Scale and Mineral Deposition Challenges
In the fifth and final part of this series we will examine issues related to mineral deposition and scale formation in cooling systems. A dominant issue for systems with cooling towers is that the water evaporation which provides most of the cooling increases the dissolved (and suspended) solids concentration. Seemingly benign make-up water may develop significant scaling potential from being “cycled up” in the tower.
Disclaimer
This article offers general information and should not serve as a design specification. Every project has unique aspects that must be individually evaluated by experts from reputable water treatment and engineering firms. Also, any issues that could potentially have an environmental influence, for example, wastewater discharge from a proposed makeup, process, or cooling water treatment system, must be presented to and approved by the proper environmental regulators during the project design phase.
References
- Post, R., Buecker, B., and Shulder, S., “Power Plant Cooling Water Fundamentals”; pre-workshop seminar for the 37th Annual Electric Utility Chemistry Workshop, June 6-8, 2017, Champaign, Illinois.
- Information provided by Dan Janikowski (ret.), materials expert with Plymouth Tube Company, Warrenville, IL, USA.
- P. Robrege, Corrosion Basics: An Introduction, Third Edition; National Association of Corrosion Engineers (now the Association for Materials Protection and Performance), Houston, Texas, 2018.
- L.S. Van Delinder, ed., Corrosion Basics: An Introduction; National Association of Corrosion Engineers (now the Association for Materials Protection and Performance), Houston, Texas, 1984.
- “Treatment of Galvanized Cooling Towers to Prevent White Rust” CTI – Guideline ESG-142 (94), Cooling Technology Institute, 1994.
- Baltimore Aircoil Company, Product & Application Handbook, Volume VI.
- W.D. Callister, Jr., Materials Science and Engineering: An Introduction, 3rd Ed., John Wiley & Sons, 1994.
About the Author
Brad Buecker, SAMCO Technologies, Buecker & Associates, LLC
President, Buecker & Associates, LLC
Brad Buecker currently serves as senior technical consultant with SAMCO Technologies and is the owner of Buecker & Associates, LLC, which provides independent technical writing/marketing services. Buecker has many years of experience in or supporting the power industry, much of it in steam generation chemistry, water treatment, air quality control, and results engineering positions with City Water, Light & Power (Springfield, Illinois) and Kansas City Power & Light Company's (now Evergy) La Cygne, Kansas, station. His work also included 11 years with two engineering firms, Burns & McDonnell and Kiewit, and two years as acting water/wastewater supervisor at a chemical plant. Buecker has a B.S. in chemistry from Iowa State University with additional coursework in fluid mechanics, energy and materials balances, and advanced inorganic chemistry. He has authored or co-authored over 300 articles for various technical trade magazines, and he has written three books on power plant chemistry and air pollution control. He is a member of AMPP, ACS, AIChE, AIST, ASME, AWT, CTI, and he is active with Power-Gen International, the Electric Utility & Cogeneration Chemistry Workshop, and the International Water Conference.