Cooling Towers: Corrosion Mechanisms to Critical Failures — Part 3
What You’ll Learn
1. Corrosion Mechanisms Are Diverse and Often Localized
While general corrosion can be expected and designed for, localized corrosion — such as pitting, microbiologically influenced corrosion (MIC), and oxygen-induced tuberculation — can lead to rapid and unexpected equipment failure.
2. Materials Selection Is Critical to Preventing Corrosion
Common assumptions about materials — such as the universal reliability of stainless steel — are flawed. Chloride levels, sulfide contamination and oxygen availability must be factored into materials choices, especially in systems with evaporative cycling like cooling towers.
3. Treatment and Design Must Account for Chemical and Biological Factors
Cooling system corrosion is affected by more than just metal type — impurities, microbial activity, and even residuals from manufacturing can trigger failures. Vigilant design review, treatment strategies and post-manufacturing processes are essential.
Cooling systems are fabricated from a variety of materials. Due to its high strength and low cost compared to other metals, carbon steel is typical for main piping to and from the cooling tower and many branch lines in the plant. Stainless steel is a common alloy for the tubes in shell-and-tube heat exchangers and is often the primary choice for plate-and-frame heat exchangers, although copper alloys, with their excellent heat-transfer properties, still find wide use.
Other metals that may appear to a lesser extent in cooling systems include aluminum and titanium as well as specialty alloys for highly corrosive fluids.
Non-metallic components include concrete basins for large cooling towers, and, although less common than in the past, wood for the tower framework.
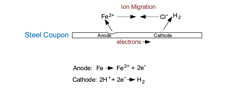
Metallic corrosion is an electrochemical process, although in some cases such as erosion-corrosion and cavitation, it is influenced by mechanical factors. As a starting point, consider the fundamental corrosion example of a carbon steel coupon immersed in an acidic solution, in this case hydrochloric acid (HCl).
The Most Common Corrosion Mechanisms
Figure 1 illustrates a classic example, albeit in an artificial environment, of general corrosion in which anodes and cathodes constantly shift around the metal surface. General corrosion is the baseline to which water-system engineers design equipment for a lifespan of about 30 years. Unfortunately, many corrosion mechanisms are localized, where in some cases, through-wall penetration of tubes, pipes, tank walls and other structures has occurred within months and sometimes even weeks.
Proper selection of chemical treatment programs is necessary to minimize localized corrosion, but so is materials selection. For example, a common myth is that stainless steel is a cure-all for corrosion difficulties, when in fact some stainless steels will rapidly corrode in certain environments.
Oxygen Corrosion
The ferrous hydroxide (Fe(OH)2) reaction product will further react with oxygen to produce solid compounds, basically rust (hydrated ferric oxides, Fe2O3∙xH2O) that settle on the metal surfaces. It is not uncommon for these corrosion products to accumulate on top of active corrosion sites to form tubercules. Other dissolved elements/compounds such as chloride and sulfate may then accumulate underneath the tubercules to increase the corrosion potential.
Given the ready supply of oxygen via air drawn through a cooling tower and the dissolved oxygen (DO) that enters with the makeup water, a primary goal of treatment programs is protecting carbon steel from DO attack (we will examine treatment details in part 6 of this series).
Pitting
The oxygen attack outlined above is localized and can be thought of as a form of pitting. Other impurities can cause pitting.
A classic example is chloride pitting of the 304 and 316 austenitic stainless steels. These materials are very popular for heat exchanger tubes and plate-and-frame HX components. Stainless steels owe their corrosion resistance to the oxide layer that forms on the surface per the addition of 12% or more chromium to the alloy. Stainless steels need a constant supply of oxygen to maintain this protective layer. However, chloride ions can penetrate the oxide film to establish localized corrosion cells. Consider the following personal case history.
Case Study: Stainless Steel Selection Pitfalls in Power Plant Design
For several years, one of my primary tasks was to review the water treatment portion of engineer, procure, construct, or EPC, specifications for combined cycle power plants. These specs came from outside design firms. Almost without exception, the design engineers would select 304L or 316L stainless steel as the tube material for the steam surface condensers.
But in numerous instances it was clearly obvious that the design team had not considered the potential for chloride pitting.
Corrosion knowledge has advanced greatly over the years, and it is now known that even seemingly low chloride concentrations (approximately 200 mg/l for 304L and 500 mg/l for 316L) can induce pitting corrosion, especially underneath deposits.2
Because most modern cooling systems have a cooling tower at the heart, all impurities, including chloride, cycle up in concentration due to evaporation from the tower. While the makeup water chlorides may be below corrosive concentrations, the recirculating water may be well above.
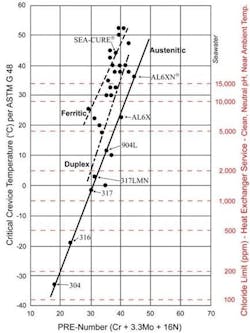
Chloride pitting resistance is frequently the primary factor in heat exchanger materials selection for water-cooled exchangers. A well-known guide is the pitting resistance equivalent number (PREN) chart as shown in Figure 4.
Higher-alloy materials beyond the 300 austenitic stainless series are recommended for cooling waters with appreciable chlorides. For highly brackish water and seawater, the ferritic and super-ferritic alloys are often recommended. Consider another important and insidious pitting example. As we will further examine in the microbiologically influenced corrosion (MIC) discussion below, sulfides can be extremely corrosive to many metals. Figure 5 shows sulfide pitting in an extracted and longitudinally cut section of a 90-10 copper-nickel condenser tube.
The tubes had been installed as a replacement for aging Admiralty brass material. Within 18 months, the new tubes failed from numerous through-wall penetrations. Investigation revealed that the tube manufacturer had used a lubricant containing sulfides during the fabrication process. They did not remove lubricant residuals following tube fabrication. Once the condenser was placed in service, the sulfides rapidly attacked the metal. A second retubing became necessary.
Microbiologically Influenced Corrosion (MIC)
Even garden-variety deposits can be problematic because they establish oxygen differential cells, where oxygen deficiency underneath a deposit causes that location to become anodic to the remaining metal. Beyond that issue, though, problems may arise from microbial metabolic processes. Sessile microbial organisms and the slime layer they produce typically harbor many organisms, including anaerobic bacteria whose metabolic processes generate corrosive compounds. A leading culprit is sulfate-reducing bacteria, such as desulfovibrio desulfuricans, which extracts oxygen from sulfate to produce hydrogen sulfide.
I directly witnessed the aftermath of a situation in which a steam condenser with over 15,000, 316L SS tubes was left open to the atmosphere, with cooling water remaining in the tubes, during a month-long scheduled maintenance outage. Upon unit startup, the plant chemists discovered severe impurity ingress to the condensate. Visual inspection revealed thousands of pinhole leaks in the tubes, forcing a unit shutdown and complete retubing of the condenser.
Other factors influence corrosion potential, including fluid velocity, surface imperfections, disruptions of metal structure and chemistry at weld locations, the list goes on. Additional details are available in the references below and from the Cooling Technology Institute. Part 4 of this series will examine issues related to mineral deposition and scale formation in cooling systems.
Disclaimer
This article offers general information and should not serve as a design specification. Every project has unique aspects that must be individually evaluated by experts from reputable water treatment and engineering firms. Also, any issues that could potentially have an environmental influence, for example, wastewater discharge from a proposed makeup, process, or cooling water treatment system, must be presented to and approved by the proper environmental regulators during the project design phase.
References
- Post, R., Buecker, B., and Shulder, S., “Power Plant Cooling Water Fundamentals”; pre-workshop seminar for the 37th Annual Electric Utility Chemistry Workshop, June 6-8, 2017, Champaign, Illinois.
- Information provided by Dan Janikowski (ret.), materials expert with Plymouth Tube Company, Warrenville, IL, USA.
About the Author
Brad Buecker, SAMCO Technologies, Buecker & Associates, LLC
President, Buecker & Associates, LLC
Brad Buecker currently serves as senior technical consultant with SAMCO Technologies and is the owner of Buecker & Associates, LLC, which provides independent technical writing/marketing services. Buecker has many years of experience in or supporting the power industry, much of it in steam generation chemistry, water treatment, air quality control, and results engineering positions with City Water, Light & Power (Springfield, Illinois) and Kansas City Power & Light Company's (now Evergy) La Cygne, Kansas, station. His work also included 11 years with two engineering firms, Burns & McDonnell and Kiewit, and two years as acting water/wastewater supervisor at a chemical plant. Buecker has a B.S. in chemistry from Iowa State University with additional coursework in fluid mechanics, energy and materials balances, and advanced inorganic chemistry. He has authored or co-authored over 300 articles for various technical trade magazines, and he has written three books on power plant chemistry and air pollution control. He is a member of AMPP, ACS, AIChE, AIST, ASME, AWT, CTI, and he is active with Power-Gen International, the Electric Utility & Cogeneration Chemistry Workshop, and the International Water Conference.