Process Puzzler: Scrub Successfully And Safely
This Month’s Puzzler
A safety review has discovered serious flaws in our relief valve vent system and electrical area classification. The reliefs for our ammonia (NH3) compressors vent on to the roof of a busy area in our plant. In addition, the compressor building lacks an exhaust fan, which makes it a Class 1, Div. 1, Group D zone.
I designed a 4-ft diameter scrubber with the relief vents going to a basin. I proposed 10% sulfuric acid (H2SO4). The superintendent doesn’t like operators working with acid. He also complains the scrubber will be ignored: the ammonia alarm goes off about six-to-ten times a year (when someone actually takes note of it). The safety director gripes about having to resize the relief valves for the pressure drop: it’s a 300-psig system! He’s also concerned the ammonia relief flows will blow right through the basin without being captured. The project group is troubled over the cost of the scrubber because of material selection.
Now, the superintendent has talked corporate into the idea of using the scrubber to handle the formaldehyde and methanol that escapes our storage tanks when we do inspections and repairs. We have a thermal oxidizer (TOX) but it only captures about 90% of the vapor from the plant.
Are there any options other than sulfuric that would lower the project cost? How much of a concern is resizing the relief valves? Can you suggest other ideas to meet the scope of this project?
Use Multiple Approaches
Scrub the NH3 with water to see if it provides sufficient control. Solubility is not a problem; if kinetics are slow, that might be addressed with engineering.
For formaldehyde and methanol vapors, you might find success with drums of activated carbon.
Andrew Yeung, R&D scientist,
Afton Chemical Corp., Pasadena, Texas
Choose A Different Solvent
Consider the following:
1. NH3 scrubbing solvent needs to be relatively safe and cost-effective. Although H2SO4 is a widely used scrubber solvent, other choices include phosphoric acid and nitric acid. Phosphoric acid is not as severe an inhalation hazard as H2SO4 and is not considered a carcinogen. However, it is not hazard-free, and demands proper handling procedures and use of personal protective equipment. In addition, it likely would not match the operating cost-effectiveness of H2SO4.
2. Strictly from a safety vantage point, citric acid is an appropriate scrubbing medium. However, its consumption rate will exceed that of H2SO4. Similarly, because NH3 has a high solubility in water, you could consider cold water as a scrubbing medium but, unlike acids which convert NH3 into salts, ammoniated water could pose odor and regulatory compliance issues.
3. Avoid piping the compressor relief valve vent directly to the scrubber because high velocity from the relief valve could damage the scrubber internals. Consider an intermediate vessel to stabilize flow and pressure. Arrange gas entry to the scrubber to avoid striking internals such as the bed support and packing at high velocity.
4. Address all findings of process hazard assessments per regulatory requirements. While the problem does not cite the findings, in general for relief valves, a quick checklist would include, for example, inlet piping and pressure drop (3% limit), back pressure and type of relief valve (pilot operated or non-pilot), and pressure release scenarios to ensure relief valves have adequate capacity to handle likely events. API-520 (October 2020) provides guidelines for sizing and installation of relief valves.
5. Repurposing the scrubber for the oxidizer vapors containing formaldehyde requires consideration of flow rate, formaldehyde content, solvent concentration, packing height equivalent to a theoretical plate, and temperature. Plastic packing has an obvious temperature limitation — so the exhaust from the oxidizer will need to be cooled. You must check velocity and pressure drop to see if the 4-ft diameter scrubber is adequate. Depending on the regulatory requirements, you may need to install an online analyzer for formaldehyde in the vent exiting the scrubber. In addition, you will need to identify a suitable scrubbing medium for formaldehyde; this could include alkaline urea, sodium meta bisulfite, and the like. Finally, you must consider disposal of the effluent from the scrubber. This may turn out to be problematic. If so, check if you can modify the oxidizer operation (temperature, vapor distribution, etc.) to improve destruction efficiency of formaldehyde and achieve environmental compliance.
G.C. Shah, consultant,
Houston
Scrub One Chemical At A Time
The trouble with most scrubbers is that the product of the chemical reaction between the solvent and the absorbent can be reversed or produces something that is also dangerous.
Consider what NH3 produces when it reacts with H2SO4: ammonium sulfate. Sulfate can be used as a fertilizer — projects have been proposed by power companies to recover scrubber waste.
Now, consider what happens when formaldehyde reacts with acid. The reaction is an acid-catalyzed Cannizzaro reaction producing formic acid and methanol. However, it’s much more complicated because multiple carbon species are present. I prefer to call the results “muck.”
The methanol will react with H2SO4 in a saponification (soap) reaction to create an ester — again, “muck.”
So, in effect, using one scrubber to handle all these compounds probably won’t work out well. However, ammonium sulfate has some advantages: stick to scrubbing ammonia alone. Because you can burn the carbon compounds in the TOX you must have on site, leave it to the TOX; for that matter, a TOX can handle NH3.
As for resizing of the relief valves: “Bill me, should be your answer.” The pressure drop only will be about 2–3 psig maximum, which is less than 1% of the set pressure. You shouldn’t be concerned about the relief vent losses until they rise to about 10% of the set pressure.
The risk of only blowing through the basin is real. I suggest something creative: put 10% glass-filled packing in the basin to add some resistance to the NH3. Add a bed limiter to the top of the packing. Use the pressure drop you would get from packed scrubbing as the pressure drop adder for the relief sizing: ~ 5–10 IWC. With a large scrubber basin, the relief flows become negligible.
I considered alternatives to H2SO4 as the solvent. Handling H2SO4 is risky, a pure ammonium sulfate is marketable as a fertilizer and will react to form a stable product with methanol and formaldehyde if you can strain out the muck. I have a safer alternative: acetic acid; ammonium acetate is marketable as a fertilizer but I doubt it will react efficiently with formaldehyde or methanol.
The gas-phase volumetric mass-transfer coefficient, Kga, for these solvents, water, acetic acid and H2SO4 will be about 2, 4, and 12, respectively in lb-mole/hr×ft2×atm. A 1–2% H2SO4 solution works well with a constant pH, although you may need a stronger solution to achieve a high Kga. The water value is the minimum. You could use the acetic acid at a much higher acid concentration without fear of heat of dilution.
In summary, the Swiss-army knife solution proposed by operations is a bad idea: stick to scrubbing one chemical at a time.
Dirk Willard, consultant,
Wooster, Ohio
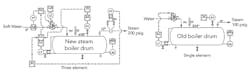
Figure 1. After addition of new boiler, operation has suffered a variety of issues.
October’s Puzzler
As part of our massive plant expansion, we added a new 200-psig boiler to our 30-year-old boiler. ASME inspectors de-rated our old boiler to 100 psig (see Figure 1).
We’ve had nothing but trouble. The relief valve blew on the new boiler shortly after start-up. Even the old boiler is operating strangely with surges in the steam flow; its mud drum has a troubled history of corrosion.
We have a new steam-flow measurement for the old boiler that comes from a vortex-shedder-type meter. (Corporate wanted a Coriolis device but it was too expensive.) The meter gave a false high reading initially but then settled down; there is an unsettling variation in the reading. The flow measurement for the new boiler surged initially but also then stabilized. Rumor has it that one of our old operators did something. We convert the steam measurements from both boilers into mass measurements. The material balance for the new boiler doesn’t make sense with the feedwater.
Another issue is the oscillation in the pressure transmitter reading on the new boiler. This has caused some disturbances in the drum level measurement and material balance.
What can we do to get the boilers operating as reliably as the old boiler? Is there a boiler control issue we should be concerned about?
Send us your comments, suggestions or solutions for this question by September 9, 2022. We’ll include as many of them as possible in the October 2022 issue and all on ChemicalProcessing.com. Send visuals — a sketch is fine. E-mail us at [email protected] or mail to Process Puzzler, Chemical Processing, 1501 E. Woodfield Rd., Suite 400N, Schaumburg, IL 60173. Fax: (630) 467-1120. Please include your name, title, location and company affiliation in the response.
And, of course, if you have a process problem you’d like to pose to our readers, send it along and we’ll be pleased to consider it for publication.