A new redox catalyst and technique can more efficiently convert ethane — a byproduct of shale gas production — into ethylene, report researchers at North Carolina State University (NC State), Raleigh. The discovery, they say, could help drastically reduce ethylene production costs and cut related carbon dioxide emissions anywhere from 60–87%.
The approach, detailed recently in an article in Science Advances, integrates ethane conversion and air separation at temperatures between 650°C and 700°C using a molten-carbonate-promoted mixed metal oxide catalyst. Current conversion methods require temperatures higher than 800°C.
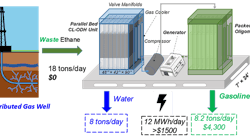
“The problem with current techniques is that you can’t scale them down to a size that makes sense for remote energy extraction sites,” says Fanxing Li, associate professor in NC State’s Department of Chemical Engineering. “With our catalyst and conversion technique, we think it would be cost effective to convert that ethane into ethylene. The ethylene could then be converted into liquid fuel, which is much easier to transport,” he adds.
Ethane outputs of 200 ft3/min or higher would make such a conversion economic, the team believes. “Our technique would require an initial investment in the installation of new, modular chemical reactors, but the jump in efficiency and ability to convert stranded ethane would be significant,” Li streses.
Figure 1. The image shows the reaction pathways for oxidative dehydrogenation of ethane facilitated by the molten carbonate shell modified perovskite redox catalyst. Source: Fanxing Li/North Carolina State University.
To determine its robustness, the team recently tested the catalyst in a larger packed bed reactor continuously for over 450 hours. “We did not see degradation on catalyst performance, confirming its robustness and potential for industrially relevant operating conditions,” notes Li.
In addition, the presence of CO2 or other hydrocarbons won't affect catalyst performance, say the researchers. “We have not tested the effect of sulfur so far but would anticipate moderate sulfur tolerance (tens of ppm H2S) for these oxides based on our past experience. The air regeneration step can help to remove sulfur poisons. The CL-ODH system would need to be operated after the sulfur polishing step,” adds Li.
The team does not foresee any major operational issues with using the molten-carbonate-promoted catalyst in remote locations. “Potential risks to address through follow up R&D include catalyst stability (tested 400 hours so far but obviously needs further testing for industrial applications) and long-term reliability of switching valves for the modular reactor. In addition, the potential corrosion of the reactor materials by the molten salt. However, these engineering challenges can be overcome based on our research results to date,” Li explains.
While the method to produce the catalyst is fairly scalable, Li discloses two potential challenges: “a. the mixed oxides preparation requires intimate interaction among various cations and high temperature annealing under oxidizing conditions. However, standard methods exist for preparing such complex oxides. Our study indicates that solids state or slurry mixing of precursors or spray drying followed with annealing in air would be adequate; b. the promoter used is a common commodity chemical and addition of promoter via impregnation is a fairly standard approach for commercial catalyst synthesis. Our research to date indicates that the thin molten salt layer on the oxide particles do not affect its handling and use in the packed bed reactor testings.”
Catalytic and Redox Solutions, LLC (CRS), an NC State-based startup co-founded by Li and Luke Neal, research professor, has licensed the technology for further development. “CRS has had initial discussions with a few potential partners, and we are open and excited to collaborate with other interested industrial partners. …We are confident the technology can be deployed within the next 5–6 years, assuming we have the right partners and support,” says Li.